
NEI intramural researchers participating in the first round of "Innovate Together" include (from left): Joanne Li, Ph.D.; Andrea Barabino, Ph.D.; John Ball, Ph.D.; Vineeta Das, Ph.D.; Ali Otadi; Gleysin Cabrera Herrera, Ph.D.; and Ruchi Sharma, Ph.D.
John Ball, Ph.D., staff scientist in the NEI Retinal Neurophysiology Section, has long wanted to visualize the functional circuitry of individual cone photoreceptors, the color-sensing neurons in the eye. Now, thanks to advances in artificial intelligence (AI) and a new NEI intramural grant program, he’ll get his chance.
The seven projects funded by the program’s first round will receive a total of $350K. The program, called “Innovate Together” enables intramural postdoctoral fellows and staff scientists to explore new tools and techniques. It also provides career-building experience in grant proposal writing and budget management independent of involvement from primary investigators.
“AI, big data analysis, high-throughput screening, and other methods blossomed after most grantees finished graduate school,” said Kapil Bharti, Ph.D., scientific director at the NEI. “This program encourages intramural researchers to get outside their comfort zone. One grantee, accustomed to working with animal models, will be working with organoids. A group that usually works with organoids is collaborating with clinicians and using AI to combine clinical and basic science data. Another project is developing tools that the entire institute can use.”
With his grant, Ball will use cloud-based AI to segment and digitally reconstruct the structure of neurons within a very large 3D electron microscopy image. The data will come from a block of retina from the thirteen-lined ground squirrel, a model for investigating cone-based eye diseases, a type of photoreceptor used for color vision.
“One of the challenges in neuroscience is that everything is so tightly packed together and interacting in complicated ways. It’s difficult to tell what you’re looking at. 3D reconstruction of neuronal wiring from serial electron microscopy is a technique that’s been around for decades, but automation and computers have increased the capability to explore these huge wiring diagrams to figure things out,” he said.
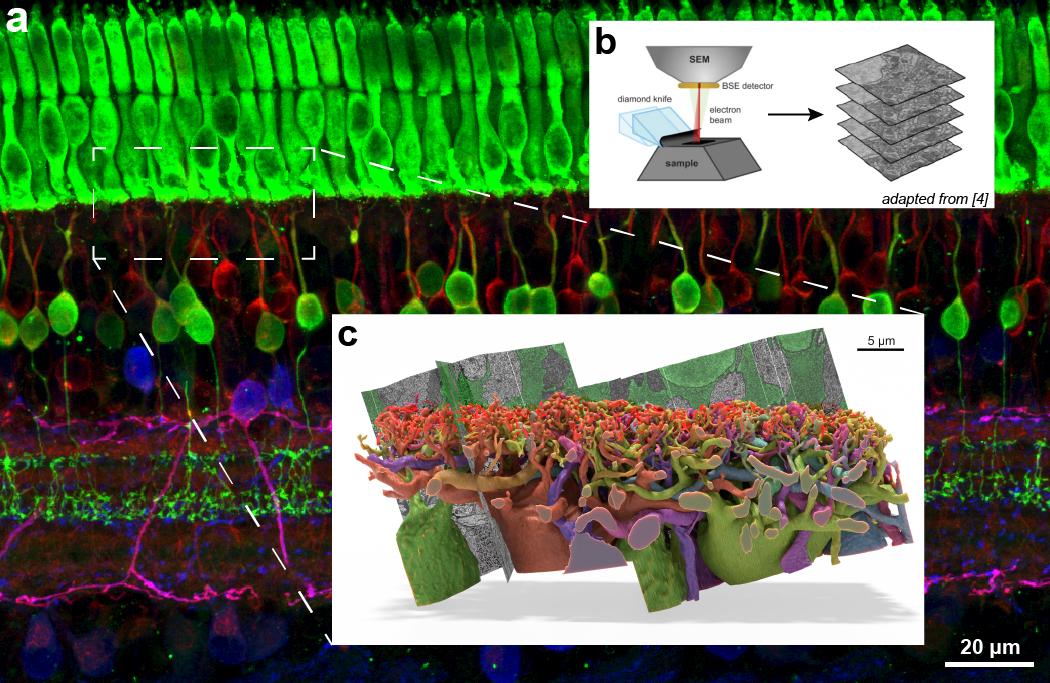
Full fluorescent image background (a) is representative of the size and scope of the 3D serial electron microscopy dataset Ball aspires to acquire, and the boxed region (c) is the approximate size of the small dataset he acquired a decade ago and which took hundreds of hours of manual labor to segment. B is a diagram showing the process of acquiring the 3D image data from a sample.
Gleysin Cabrera Herrera, Ph.D., a postdoctoral fellow in the NEI Laboratory of Retinal Cell and Molecular Biology, specializes in the characterization of carbohydrates and proteins using mass spectrometry, but she wants to learn how to interpret genomics data. Her grant supports the search for biomarkers of early age-related macular degeneration (AMD) by interpreting findings from AMD population genome-wide association studies (GWAS) and corresponding quantitative proteomics data.
For Andrea Barabino, Ph.D., a postdoc in the Ophthalmic Genetics and Visual Functions Branch (OVGFB), the IRP grant will give him the chance to use novel approach to study how two key retinal cells work together.
The back of the eye is like a camera with sensors (photoreceptors) that capture light to make images and batteries (retinal pigment epithelial cells) for energy. In eye diseases, like age-related macular degeneration (AMD), these two cell types stop working well together, leading to vision loss.
“Scientists have tried to study these cells together in the lab, but it’s been challenging because these cells don’t stick together well outside the body,” said Barabino.
He and Ali Otadi, a postbac in OVGFB, proposed using stem cells, 3D printing, and unique proteins that act like glue to get the RPE and photoreceptors to stick together. “Bringing together RPE and photoreceptors in vitro in a more physiologically relevant system could help us understand how these cells interact in healthy and diseased eyes and could lead to new treatments,” he said.
With her grant, Ruchi Sharma, Ph.D., staff scientist in OVGFB, is forming a multidisciplinary team that will use AI and machine learning (ML) to find meaningful connections between disease-in-a-dish lab models and clinical research involving patients with AMD.
“I have always loved the idea of a multi-disciplinary team that brings together clinicians, big data scientists and experts in AI and ML, and this funding opportunity has given me the chance to make that happen,” said Sharma.
Joanne Li, Ph.D., is stepping outside her comfort zone as a biomedical engineer and using the grant to collaborate with Vineeta Das, Ph.D., a postdoc with expertise developing AI-based image analysis methods (both in OGVFB). Together, they plan to develop a system that combines high-resolution optical coherence tomography imaging and an AI-based analysis platform to detect the earliest sign of age-related diseases.
“By having a system that images living human eyes at the cellular level and systematically analyzes and tracks changes over time, we hope to understand how the ‘normal’ path of aging deviates in diseases such as AMD,” Li said.
“Scientists tend to stick with what they are good at. The NEI IRP grant program challenges us to think outside our area of expertise and, more importantly, gives us funding to pursue those ideas,” Herrera said.