Diazoxide for Severe or Recurrent Neonatal Hypoglycemia: A Randomized Clinical Trial
- PMID: 38869900
- PMCID: PMC11177163
- DOI: 10.1001/jamanetworkopen.2024.15764
Diazoxide for Severe or Recurrent Neonatal Hypoglycemia: A Randomized Clinical Trial
Abstract
Importance: Neonatal hypoglycemia is an important preventable cause of neurodevelopmental impairment, but there is a paucity of evidence to guide treatment.
Objective: To evaluate whether early, low-dose oral diazoxide for severe or recurrent neonatal hypoglycemia reduces time to resolution of hypoglycemia.
Design, setting, and participants: This 2-arm, placebo-controlled randomized clinical trial was conducted from May 2020 to February 2023 in tertiary neonatal units at 2 New Zealand hospitals. Participants were neonates born at 35 or more weeks' gestation and less than 1 week of age with severe hypoglycemia (blood glucose concentration <22 mg/dL or <36 mg/dL despite 2 doses of dextrose gel) or recurrent hypoglycemia (≥3 episodes of a blood glucose concentration <47 mg/dL within 48 hours).
Interventions: Newborns were randomized 1:1 to receive diazoxide suspension (loading dose, 5 mg/kg; maintenance, 1.5 mg/kg every 12 hours) or placebo, titrated per protocol.
Main outcome and measures: The primary outcome was time to resolution of hypoglycemia, defined as enteral bolus feeding without intravenous fluids and normoglycemia (blood glucose concentration of 47-98 mg/dL) for at least 24 hours, compared between groups using adjusted Cox proportional hazards regression. Hazard ratios adjusted for stratification variables and gestation length are reported. Prespecified secondary outcomes, including number of blood glucose tests and episodes of hypoglycemia, duration of hypoglycemia, and time to enteral bolus feeding and weaning from intravenous fluids, were compared by generalized linear models. Newborns were followed up for at least 2 weeks.
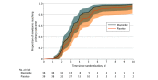
Results: Of 154 newborns screened, 75 were randomized and 74 with evaluable data were included in the analysis (mean [SD] gestational age for the full cohort, 37.6 [1.6] weeks), 36 in the diazoxide group and 38 in the placebo group. Baseline characteristics were similar: in the diazoxide group, mean (SD) gestational age was 37.9 (1.6) weeks and 26 (72%) were male; in the placebo group, mean (SD) gestational age was 37.4 (1.5) weeks and 27 (71%) were male. There was no significant difference in time to resolution of hypoglycemia (adjusted hazard ratio [AHR], 1.39; 95% CI, 0.84-2.23), possibly due to increased episodes of elevated blood glucose concentration and longer time to normoglycemia in the diazoxide group. Resolution of hypoglycemia, when redefined post hoc as enteral bolus feeding without intravenous fluids for at least 24 hours with no further hypoglycemia, was reached by more newborns in the diazoxide group (AHR, 2.60; 95% CI, 1.53-4.46). Newborns in the diazoxide group had fewer blood glucose tests (adjusted count ratio [ACR], 0.63; 95% CI, 0.56-0.71) and episodes of hypoglycemia (ACR, 0.32; 95% CI, 0.17-0.63), reduced duration of hypoglycemia (adjusted ratio of geometric means [ARGM], 0.18; 95% CI, 0.06-0.53), and reduced time to enteral bolus feeding (ARGM, 0.74; 95% CI, 0.58-0.95) and weaning from intravenous fluids (ARGM, 0.72; 95% CI, 0.60-0.87). Only 2 newborns (6%) treated with diazoxide had hypoglycemia after the loading dose compared with 20 (53%) with placebo.
Conclusions and relevance: In this randomized clinical trial, early treatment of severe or recurrent neonatal hypoglycemia with low-dose oral diazoxide did not reduce time to resolution of hypoglycemia but reduced time to enteral bolus feeding and weaning from intravenous fluids, duration of hypoglycemia, and frequency of blood glucose testing compared with placebo.
Trial registration: ANZCTR.org.au Identifier: ACTRN12620000129987.
Conflict of interest statement
Figures
Similar articles
-
Oral diazoxide versus placebo for severe or recurrent neonatal hypoglycaemia: Neonatal Glucose Care Optimisation (NeoGluCO) study - a randomised controlled trial.BMJ Open. 2022 Aug 17;12(8):e059452. doi: 10.1136/bmjopen-2021-059452. BMJ Open. 2022. PMID: 35977769 Free PMC article. Clinical Trial.
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
Association of Neonatal Glycemia With Neurodevelopmental Outcomes at 4.5 Years.JAMA Pediatr. 2017 Oct 1;171(10):972-983. doi: 10.1001/jamapediatrics.2017.1579. JAMA Pediatr. 2017. PMID: 28783802 Free PMC article.
-
When it is more than transient neonatal hypoglycemia: hyperinsulinemia--a case study challenge.Neonatal Netw. 2001 Jun;20(4):5-11. doi: 10.1891/0730-0832.20.4.5. Neonatal Netw. 2001. PMID: 12143902 Review.
-
Oral dextrose gel to prevent hypoglycaemia in at-risk neonates.Cochrane Database Syst Rev. 2023 Nov 28;11(11):CD012152. doi: 10.1002/14651858.CD012152.pub4. Cochrane Database Syst Rev. 2023. PMID: 38014716 Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

