Heat shock protein: a double-edged sword linking innate immunity and hepatitis B virus infection
- PMID: 37128472
- PMCID: PMC10148040
- DOI: 10.1016/j.jve.2023.100322
Heat shock protein: a double-edged sword linking innate immunity and hepatitis B virus infection
Abstract
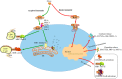
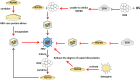
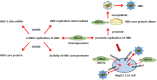
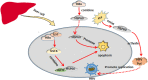
Heat shock proteins (HSPs), which have a variety of functions, are one of the stress protein families. In recent years, They have been reported to play a dual role in hepatitis B virus (HBV) which as persistent infection which is associated with, cirrhosis and liver cancer. In this article, we have summarized the regulatory mechanisms between HSPs and viruses, especially HBV and associated diseases based on HSP biological functions of in response to viral infections. In view of their potential as broad-spectrum antiviral targets, we have also discuss current progress and challenges in drug development based on HSPs, as well as the potential applications of agents that have been evaluated clinically in HBV treatment.
Keywords: Antiviral; HSPs; Hepatitis B virus; Molecular chaperones; Vaccine adjuvant.
© 2023 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
Stress proteins: the biological functions in virus infection, present and challenges for target-based antiviral drug development.Signal Transduct Target Ther. 2020 Jul 13;5(1):125. doi: 10.1038/s41392-020-00233-4. Signal Transduct Target Ther. 2020. PMID: 32661235 Free PMC article. Review.
-
Mode of Action of Heat Shock Protein (HSP) Inhibitors against Viruses through Host HSP and Virus Interactions.Genes (Basel). 2023 Mar 25;14(4):792. doi: 10.3390/genes14040792. Genes (Basel). 2023. PMID: 37107550 Free PMC article. Review.
-
Structure, gene expression, and putative functions of crustacean heat shock proteins in innate immunity.Dev Comp Immunol. 2021 Feb;115:103875. doi: 10.1016/j.dci.2020.103875. Epub 2020 Sep 25. Dev Comp Immunol. 2021. PMID: 32987013 Review.
-
NK cells: a double-edged sword in chronic hepatitis B virus infection.Front Immunol. 2013 Mar 1;4:57. doi: 10.3389/fimmu.2013.00057. eCollection 2013. Front Immunol. 2013. PMID: 23459859 Free PMC article.
-
Heat Shock Protein (HSP) Drug Discovery and Development: Targeting Heat Shock Proteins in Disease.Curr Top Med Chem. 2016;16(25):2753-64. doi: 10.2174/1568026616666160413141911. Curr Top Med Chem. 2016. PMID: 27072696 Free PMC article. Review.
Cited by
-
Crossroad between the Heat Shock Protein and Inflammation Pathway in Acquiring Drug Resistance: A Possible Target for Future Cancer Therapeutics.Biomedicines. 2023 Sep 26;11(10):2639. doi: 10.3390/biomedicines11102639. Biomedicines. 2023. PMID: 37893013 Free PMC article. Review.
-
USP14 Positively Modulates Head and Neck Squamous Carcinoma Tumorigenesis and Potentiates Heat Shock Pathway through HSF1 Stabilization.Cancers (Basel). 2023 Sep 1;15(17):4385. doi: 10.3390/cancers15174385. Cancers (Basel). 2023. PMID: 37686660 Free PMC article.
References
-
- Huang Y-y. Hubei University Of Technology; 2016. Study on Anti-HBV Drugs Targeting Heat Shock Protein.
-
- Yang Y.-X., Huang J.-P., Li S.-N., Li J., Ling T., Xie T., Xu L.-G. HSPBP1 facilitates cellular RLR-mediated antiviral response by inhibiting the K48-linked ubiquitination of RIG-I. Mol Immunol. 2021;134:62–71. - PubMed
-
- Zhang W.J., Wang R.Q., Li L.T., Fu W., Chen H.C., Liu Z.F. Hsp90 is involved in pseudorabies virus virion assembly via stabilizing major capsid protein VP5. Virology. 2021;553:70–80. - PubMed
-
- Wang X-h, Xu W-h, Zhang X-l, Ma D-y, Lei Y-f, Zheng L., Guo D-x, Wu Z-j, Zhang H., Cao H-w. Effect of PEDV protein on expression of GRP78, the key protein of UPR response in host cells. J Heilongjiang Bayi Agric Univ. 2021;33(4):60–66.
Publication types
LinkOut - more resources
Full Text Sources

