AG129 Mice as a Comprehensive Model for the Experimental Assessment of Mosquito Vector Competence for Arboviruses
- PMID: 36015000
- PMCID: PMC9412449
- DOI: 10.3390/pathogens11080879
AG129 Mice as a Comprehensive Model for the Experimental Assessment of Mosquito Vector Competence for Arboviruses
Abstract
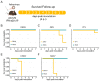
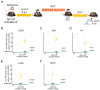
Arboviruses (an acronym for "arthropod-borne virus"), such as dengue, yellow fever, Zika, and Chikungunya, are important human pathogens transmitted by mosquitoes. These viruses impose a growing burden on public health. Despite laboratory mice having been used for decades for understanding the basic biological phenomena of these viruses, it was only recently that researchers started to develop immunocompromised animals to study the pathogenesis of arboviruses and their transmission in a way that parallels natural cycles. Here, we show that the AG129 mouse (IFN α/β/γ R-/-) is a suitable and comprehensive vertebrate model for studying the mosquito vector competence for the major arboviruses of medical importance, namely the dengue virus (DENV), yellow fever virus (YFV), Zika virus (ZIKV), Mayaro virus (MAYV), and Chikungunya virus (CHIKV). We found that, after intraperitoneal injection, AG129 mice developed a transient viremia lasting several days, peaking on day two or three post infection, for all five arboviruses tested in this study. Furthermore, we found that the observed viremia was ample enough to infect Aedes aegypti during a blood meal from the AG129 infected mice. Finally, we demonstrated that infected mosquitoes could transmit each of the tested arboviruses back to naïve AG129 mice, completing a full transmission cycle of these vector-borne viruses. Together, our data show that A129 mice are a simple and comprehensive vertebrate model for studies of vector competence, as well as investigations into other aspects of mosquito biology that can affect virus-host interactions.
Keywords: AG129; arbovirus; mice model; vector competence; vertebrate transmission model; virus transmission.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Suitable Mouse Model to Study Dynamics of West Nile Virus Infection in Culex quinquefasciatus Mosquitoes.Trop Med Infect Dis. 2024 Sep 2;9(9):201. doi: 10.3390/tropicalmed9090201. Trop Med Infect Dis. 2024. PMID: 39330890 Free PMC article.
-
Minireview: Epidemiological impact of arboviral diseases in Latin American countries, arbovirus-vector interactions and control strategies.Pathog Dis. 2021 Sep 6;79(7):ftab043. doi: 10.1093/femspd/ftab043. Pathog Dis. 2021. PMID: 34410378 Review.
-
Cell-Fusing Agent Virus Reduces Arbovirus Dissemination in Aedes aegypti Mosquitoes In Vivo.J Virol. 2019 Aug 28;93(18):e00705-19. doi: 10.1128/JVI.00705-19. Print 2019 Sep 15. J Virol. 2019. PMID: 31243123 Free PMC article.
-
Vector competence of Aedes aegypti from Havana, Cuba, for dengue virus type 1, chikungunya, and Zika viruses.PLoS Negl Trop Dis. 2020 Dec 3;14(12):e0008941. doi: 10.1371/journal.pntd.0008941. eCollection 2020 Dec. PLoS Negl Trop Dis. 2020. PMID: 33270652 Free PMC article.
-
Human Urban Arboviruses Can Infect Wild Animals and Jump to Sylvatic Maintenance Cycles in South America.Front Cell Infect Microbiol. 2019 Jul 17;9:259. doi: 10.3389/fcimb.2019.00259. eCollection 2019. Front Cell Infect Microbiol. 2019. PMID: 31380302 Free PMC article. Review.
Cited by
-
Comparison of Chikungunya Virus-Induced Disease Progression and Pathogenesis in Type-I Interferon Receptor-Deficient Mice (A129) and Two Wild-Type (129Sv/Ev and C57BL/6) Mouse Strains.Viruses. 2024 Sep 27;16(10):1534. doi: 10.3390/v16101534. Viruses. 2024. PMID: 39459867 Free PMC article.
-
The inoculum dose of Zika virus can affect the viral replication dynamics, cytokine responses and survival rate in immunocompromised AG129 mice.Mol Biomed. 2024 Aug 3;5(1):30. doi: 10.1186/s43556-024-00195-x. Mol Biomed. 2024. PMID: 39095588 Free PMC article.
-
Mouse Models of Mayaro Virus.Viruses. 2023 Aug 24;15(9):1803. doi: 10.3390/v15091803. Viruses. 2023. PMID: 37766210 Free PMC article. Review.
-
Recombinant Protein Mimicking the Antigenic Structure of the Viral Surface Envelope Protein Reinforces Induction of an Antigen-Specific and Virus-Neutralizing Immune Response Against Dengue Virus.J Microbiol. 2023 Jan;61(1):131-143. doi: 10.1007/s12275-023-00021-z. Epub 2023 Feb 1. J Microbiol. 2023. PMID: 36723792 Free PMC article.
-
A robust mouse model of HPIV-3 infection and efficacy of GS-441524 against virus-induced lung pathology.Nat Commun. 2024 Sep 5;15(1):7765. doi: 10.1038/s41467-024-52071-5. Nat Commun. 2024. PMID: 39237507 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

