Towards environmental detection of Chagas disease vectors and pathogen
- PMID: 35701602
- PMCID: PMC9194887
- DOI: 10.1038/s41598-022-14051-x
Towards environmental detection of Chagas disease vectors and pathogen
Abstract
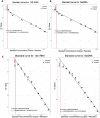
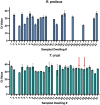
Chagas disease vector control relies on prompt, accurate identification of houses infested with triatomine bugs for targeted insecticide spraying. However, most current detection methods are laborious, lack standardization, have substantial operational costs and limited sensitivity, especially when triatomine bug densities are low or highly focal. We evaluated the use of FTA cards or cotton-tipped swabs to develop a low-technology, non-invasive method of detecting environmental DNA (eDNA) from both triatomine bugs and Trypanosoma cruzi for use in household surveillance in eastern Colombia, an endemic region for Chagas disease. Study findings demonstrated that Rhodnius prolixus eDNA, collected on FTA cards, can be detected at temperatures between 21 and 32 °C, when deposited by individual, recently blood-fed nymphs. Additionally, cotton-tipped swabs are a feasible tool for field sampling of both T. cruzi and R. prolixus eDNA in infested households and may be preferable due to their lower cost. eDNA detection should not yet replace current surveillance tools, but instead be evaluated in parallel as a more sensitive, higher-throughput, lower cost alternative. eDNA collection requires virtually no skills or resources in situ and therefore has the potential to be implemented in endemic communities as part of citizen science initiatives to control Chagas disease transmission.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Prevention of the transmission of Chagas' disease with pyrethroid-impregnated materials.Am J Trop Med Hyg. 2003 Mar;68(3):307-11. Am J Trop Med Hyg. 2003. PMID: 12685636 Clinical Trial.
-
[Attalea butyracea palms adjacent to housing as a source of infestation by Rhodnius prolixus (Hemiptera: Reduviidae)].Biomedica. 2012 Jun;32(2):277-85. doi: 10.1590/S0120-41572012000300016. Biomedica. 2012. PMID: 23242302 Spanish.
-
Eco-epidemiological study reveals the importance of Triatoma dimidiata in the Trypanosoma cruzi transmission, in a municipality certified without transmission by Rhodnius prolixus in Colombia.Acta Trop. 2020 Sep;209:105550. doi: 10.1016/j.actatropica.2020.105550. Epub 2020 May 28. Acta Trop. 2020. PMID: 32473116
-
What makes an effective Chagas disease vector? Factors underlying Trypanosoma cruzi-triatomine interactions.Acta Trop. 2018 Jul;183:23-31. doi: 10.1016/j.actatropica.2018.04.008. Epub 2018 Apr 3. Acta Trop. 2018. PMID: 29625091 Review.
-
Insecticide resistance in vector Chagas disease: evolution, mechanisms and management.Acta Trop. 2015 Sep;149:70-85. doi: 10.1016/j.actatropica.2015.05.014. Epub 2015 May 21. Acta Trop. 2015. PMID: 26003952 Review.
Cited by
-
Detection of the Endangered Siamese Bat Catfish (Oreoglanis siamensis Smith, 1933) in Doi Inthanon National Park Using Environmental DNA.Animals (Basel). 2023 Feb 3;13(3):538. doi: 10.3390/ani13030538. Animals (Basel). 2023. PMID: 36766427 Free PMC article.
-
Artificial Feeding Systems for Vector-Borne Disease Studies.Biology (Basel). 2024 Mar 15;13(3):188. doi: 10.3390/biology13030188. Biology (Basel). 2024. PMID: 38534457 Free PMC article. Review.
-
Interrogating the transmission dynamics of Trypanosoma cruzi (Trypanosomatida, Trypanosomatidae) by Triatoma venosa (Hemiptera: Reduviidae) after the elimination of vector transmission by Rhodnius prolixus in Boyacá eastern Colombia.Front Cell Infect Microbiol. 2022 Oct 6;12:998202. doi: 10.3389/fcimb.2022.998202. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36275020 Free PMC article.
-
Bacterial microbiota from the gut of Rhodnius ecuadoriensis, a vector of Chagas disease in Ecuador's Central Coast and Southern Andes.Front Microbiol. 2024 Sep 23;15:1464720. doi: 10.3389/fmicb.2024.1464720. eCollection 2024. Front Microbiol. 2024. PMID: 39376708 Free PMC article.
References
-
- Chagas disease - Level 3 cause: Institute of Health Metrics and Evaluation. http://www.healthdata.org/results/gbd_summaries/2019/chagas-disease-leve... (2019).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

