The comparative effectiveness of COVID-19 monoclonal antibodies: A learning health system randomized clinical trial
- PMID: 35697146
- PMCID: PMC9187853
- DOI: 10.1016/j.cct.2022.106822
The comparative effectiveness of COVID-19 monoclonal antibodies: A learning health system randomized clinical trial
Abstract
Background: Monoclonal antibodies (mAb) that neutralize SARS-CoV-2 decrease hospitalization and death compared to placebo in patients with mild to moderate COVID-19; however, comparative effectiveness is unknown. We report the comparative effectiveness of bamlanivimab, bamlanivimab-etesevimab, and casirivimab-imdevimab.
Methods: A learning health system platform trial in a U.S. health system enrolled patients meeting mAb Emergency Use Authorization criteria. An electronic health record-embedded application linked local mAb inventory to patient encounters and provided random mAb allocation. Primary outcome was hospital-free days to day 28. Primary analysis was a Bayesian model adjusting for treatment location, age, sex, and time. Inferiority was defined as 99% posterior probability of an odds ratio < 1. Equivalence was defined as 95% posterior probability the odds ratio is within a given bound.
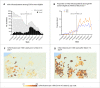
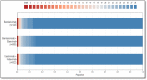
Findings: Between March 10 and June 25, 2021, 1935 patients received treatment. Median hospital-free days were 28 (IQR 28, 28) for each mAb. Mortality was 0.8% (1/128), 0.8% (7/885), and 0.7% (6/922) for bamlanivimab, bamlanivimab-etesevimab, and casirivimab-imdevimab, respectively. Relative to casirivimab-imdevimab (n = 922), median adjusted odds ratios were 0.58 (95% credible interval [CI] 0.30-1.16) and 0.94 (95% CI 0.72-1.24) for bamlanivimab (n = 128) and bamlanivimab-etesevimab (n = 885), respectively. These odds ratios yielded 91% and 94% probabilities of inferiority of bamlanivimab versus bamlanivimab-etesevimab and casirivimab-imdevimab, and an 86% probability of equivalence between bamlanivimab-etesevimab and casirivimab-imdevimab.
Interpretation: Among patients with mild to moderate COVID-19, bamlanivimab-etesevimab or casirivimab-imdevimab treatment resulted in 86% probability of equivalence. No treatment met prespecified criteria for statistical equivalence. Median hospital-free days to day 28 were 28 (IQR 28, 28) for each mAb.
Funding and registration: This work received no external funding. The U.S. government provided the reported mAb. This trial is registered at ClinicalTrials.gov, NCT04790786.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
None of the authors have conflicts of interest to report.
Figures
Similar articles
-
The UPMC OPTIMISE-C19 (OPtimizing Treatment and Impact of Monoclonal antIbodieS through Evaluation for COVID-19) trial: a structured summary of a study protocol for an open-label, pragmatic, comparative effectiveness platform trial with response-adaptive randomization.Trials. 2021 May 25;22(1):363. doi: 10.1186/s13063-021-05316-3. Trials. 2021. PMID: 34034784 Free PMC article.
-
Effect of timing of casirivimab and imdevimab administration relative to mRNA-1273 COVID-19 vaccination on vaccine-induced SARS-CoV-2 neutralising antibody responses: a prospective, open-label, phase 2, randomised controlled trial.Lancet Infect Dis. 2025 Jan;25(1):52-67. doi: 10.1016/S1473-3099(24)00421-3. Epub 2024 Sep 2. Lancet Infect Dis. 2025. PMID: 39236733 Clinical Trial.
-
Casirivimab and imdevimab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial.Lancet. 2022 Feb 12;399(10325):665-676. doi: 10.1016/S0140-6736(22)00163-5. Lancet. 2022. PMID: 35151397 Free PMC article. Clinical Trial.
-
Sotrovimab in the treatment of coronavirus disease-2019 (COVID-19): a systematic review and meta-analysis of randomized clinical trials.Naunyn Schmiedebergs Arch Pharmacol. 2024 Dec;397(12):9573-9589. doi: 10.1007/s00210-024-03298-y. Epub 2024 Jul 20. Naunyn Schmiedebergs Arch Pharmacol. 2024. PMID: 39031183 Review.
-
Accuracy of routine laboratory tests to predict mortality and deterioration to severe or critical COVID-19 in people with SARS-CoV-2.Cochrane Database Syst Rev. 2024 Aug 6;8(8):CD015050. doi: 10.1002/14651858.CD015050.pub2. Cochrane Database Syst Rev. 2024. PMID: 39105481 Free PMC article. Review.
Cited by
-
Exploratory data on the clinical efficacy of monoclonal antibodies against SARS-CoV-2 Omicron variant of concern.Elife. 2022 Nov 22;11:e79639. doi: 10.7554/eLife.79639. Elife. 2022. PMID: 36413383 Free PMC article. Clinical Trial.
-
Learning health systems on the front lines to strengthen care against future pandemics and climate change: a rapid review.BMC Health Serv Res. 2024 Jul 22;24(1):829. doi: 10.1186/s12913-024-11295-3. BMC Health Serv Res. 2024. PMID: 39039551 Free PMC article. Review.
-
Monoclonal Antibodies for Treatment of SARS-CoV-2 Infection During Pregnancy : A Cohort Study.Ann Intern Med. 2022 Dec;175(12):1707-1715. doi: 10.7326/M22-1329. Epub 2022 Nov 15. Ann Intern Med. 2022. PMID: 36375150 Free PMC article.
-
Benefits of near-universal vaccination and treatment access to manage COVID-19 burden in the United States.medRxiv [Preprint]. 2023 Feb 10:2023.02.08.23285658. doi: 10.1101/2023.02.08.23285658. medRxiv. 2023. Update in: BMC Med. 2023 Aug 24;21(1):321. doi: 10.1186/s12916-023-03025-z. PMID: 36798204 Free PMC article. Updated. Preprint.
-
Designing and Implementing "Living and Breathing" Clinical Trials: An Overview and Lessons Learned from the COVID-19 Pandemic.Crit Care Clin. 2023 Oct;39(4):717-732. doi: 10.1016/j.ccc.2023.02.002. Epub 2023 Feb 17. Crit Care Clin. 2023. PMID: 37704336 Free PMC article. Review.
References
-
- Bariola J.R., McCreary E.K., Wadas R.J., et al. Impact of bamlanivimab monoclonal antibody treatment on hospitalization and mortality among nonhospitalized adults with severe acute respiratory syndrome Coronavirus 2 infection. Open Forum Infect Dis. July 2021;8 doi: 10.1093/ofid/ofab254. ofab254. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

