Natural killer cells: a promising immunotherapy for cancer
- PMID: 35606854
- PMCID: PMC9125849
- DOI: 10.1186/s12967-022-03437-0
Natural killer cells: a promising immunotherapy for cancer
Abstract
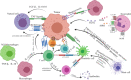
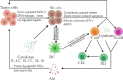
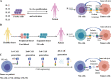
As a promising alternative platform for cellular immunotherapy, natural killer cells (NK) have recently gained attention as an important type of innate immune regulatory cell. NK cells can rapidly kill multiple adjacent cancer cells through non-MHC-restrictive effects. Although tumors may develop multiple resistance mechanisms to endogenous NK cell attack, in vitro activation, expansion, and genetic modification of NK cells can greatly enhance their anti-tumor activity and give them the ability to overcome drug resistance. Some of these approaches have been translated into clinical applications, and clinical trials of NK cell infusion in patients with hematological malignancies and solid tumors have thus far yielded many encouraging clinical results. CAR-T cells have exhibited great success in treating hematological malignancies, but their drawbacks include high manufacturing costs and potentially fatal toxicity, such as cytokine release syndrome. To overcome these issues, CAR-NK cells were generated through genetic engineering and demonstrated significant clinical responses and lower adverse effects compared with CAR-T cell therapy. In this review, we summarize recent advances in NK cell immunotherapy, focusing on NK cell biology and function, the types of NK cell therapy, and clinical trials and future perspectives on NK cell therapy.
Keywords: CAR-NK cells; Cancer; Immunotherapy; NK cells.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no conflict of interest relating to the publication of this manuscript.
Figures

Similar articles
-
CAR-NK Cells: From Natural Basis to Design for Kill.Front Immunol. 2021 Dec 14;12:707542. doi: 10.3389/fimmu.2021.707542. eCollection 2021. Front Immunol. 2021. PMID: 34970253 Free PMC article. Review.
-
Current progress of chimeric antigen receptor (CAR) T versus CAR NK cell for immunotherapy of solid tumors.Life Sci. 2024 Jan 15;337:122381. doi: 10.1016/j.lfs.2023.122381. Epub 2023 Dec 23. Life Sci. 2024. PMID: 38145710 Review.
-
Chimeric antigen receptor (CAR) natural killer (NK)-cell therapy: leveraging the power of innate immunity.Br J Haematol. 2021 Apr;193(2):216-230. doi: 10.1111/bjh.17186. Epub 2020 Nov 20. Br J Haematol. 2021. PMID: 33216984 Free PMC article. Review.
-
CAR-engineered NK cells; a promising therapeutic option for treatment of hematological malignancies.Stem Cell Res Ther. 2021 Jul 2;12(1):374. doi: 10.1186/s13287-021-02462-y. Stem Cell Res Ther. 2021. PMID: 34215336 Free PMC article. Review.
-
[Allogeneic CAR-NK cells: A promising alternative to autologous CAR-T cells - State of the art, sources of NK cells, limits and perspectives].Bull Cancer. 2021 Oct;108(10S):S81-S91. doi: 10.1016/j.bulcan.2021.06.007. Bull Cancer. 2021. PMID: 34920811 Review. French.
Cited by
-
Chimeric antigen receptor-engineered NK cells: new weapons of cancer immunotherapy with great potential.Exp Hematol Oncol. 2022 Nov 2;11(1):85. doi: 10.1186/s40164-022-00341-7. Exp Hematol Oncol. 2022. PMID: 36324149 Free PMC article. Review.
-
Parainfluenza Virus 5 V Protein Blocks Interferon Gamma-Mediated Upregulation of NK Cell Inhibitory Ligands and Improves NK Cell Killing of Neuroblastoma Cells.Viruses. 2024 Aug 9;16(8):1270. doi: 10.3390/v16081270. Viruses. 2024. PMID: 39205244 Free PMC article.
-
Extracellular vesicles derived from immortalized human natural killer cell line NK3.3 as a novel therapeutic for multiple myeloma.Front Immunol. 2023 Sep 25;14:1265101. doi: 10.3389/fimmu.2023.1265101. eCollection 2023. Front Immunol. 2023. PMID: 37818374 Free PMC article.
-
Therapeutic approaches to enhance natural killer cell cytotoxicity.Front Immunol. 2024 Mar 11;15:1356666. doi: 10.3389/fimmu.2024.1356666. eCollection 2024. Front Immunol. 2024. PMID: 38545115 Free PMC article. Review.
-
A bibliometric and scientific knowledge-map study of the chimeric antigen receptor (CAR) natural killer (NK) cell-related research from 2010 to 2022.Front Immunol. 2022 Aug 12;13:969196. doi: 10.3389/fimmu.2022.969196. eCollection 2022. Front Immunol. 2022. PMID: 36032149 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

