Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study
- PMID: 35305296
- PMCID: PMC8926413
- DOI: 10.1016/S0140-6736(22)00462-7
Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study
Abstract
Background: The omicron variant (B.1.1.529) of SARS-CoV-2 has demonstrated partial vaccine escape and high transmissibility, with early studies indicating lower severity of infection than that of the delta variant (B.1.617.2). We aimed to better characterise omicron severity relative to delta by assessing the relative risk of hospital attendance, hospital admission, or death in a large national cohort.
Methods: Individual-level data on laboratory-confirmed COVID-19 cases resident in England between Nov 29, 2021, and Jan 9, 2022, were linked to routine datasets on vaccination status, hospital attendance and admission, and mortality. The relative risk of hospital attendance or admission within 14 days, or death within 28 days after confirmed infection, was estimated using proportional hazards regression. Analyses were stratified by test date, 10-year age band, ethnicity, residential region, and vaccination status, and were further adjusted for sex, index of multiple deprivation decile, evidence of a previous infection, and year of age within each age band. A secondary analysis estimated variant-specific and vaccine-specific vaccine effectiveness and the intrinsic relative severity of omicron infection compared with delta (ie, the relative risk in unvaccinated cases).
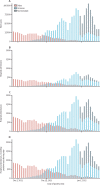
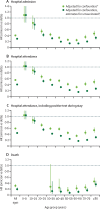
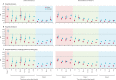
Findings: The adjusted hazard ratio (HR) of hospital attendance (not necessarily resulting in admission) with omicron compared with delta was 0·56 (95% CI 0·54-0·58); for hospital admission and death, HR estimates were 0·41 (0·39-0·43) and 0·31 (0·26-0·37), respectively. Omicron versus delta HR estimates varied with age for all endpoints examined. The adjusted HR for hospital admission was 1·10 (0·85-1·42) in those younger than 10 years, decreasing to 0·25 (0·21-0·30) in 60-69-year-olds, and then increasing to 0·47 (0·40-0·56) in those aged at least 80 years. For both variants, past infection gave some protection against death both in vaccinated (HR 0·47 [0·32-0·68]) and unvaccinated (0·18 [0·06-0·57]) cases. In vaccinated cases, past infection offered no additional protection against hospital admission beyond that provided by vaccination (HR 0·96 [0·88-1·04]); however, for unvaccinated cases, past infection gave moderate protection (HR 0·55 [0·48-0·63]). Omicron versus delta HR estimates were lower for hospital admission (0·30 [0·28-0·32]) in unvaccinated cases than the corresponding HR estimated for all cases in the primary analysis. Booster vaccination with an mRNA vaccine was highly protective against hospitalisation and death in omicron cases (HR for hospital admission 8-11 weeks post-booster vs unvaccinated: 0·22 [0·20-0·24]), with the protection afforded after a booster not being affected by the vaccine used for doses 1 and 2.
Interpretation: The risk of severe outcomes following SARS-CoV-2 infection is substantially lower for omicron than for delta, with higher reductions for more severe endpoints and significant variation with age. Underlying the observed risks is a larger reduction in intrinsic severity (in unvaccinated individuals) counterbalanced by a reduction in vaccine effectiveness. Documented previous SARS-CoV-2 infection offered some protection against hospitalisation and high protection against death in unvaccinated individuals, but only offered additional protection in vaccinated individuals for the death endpoint. Booster vaccination with mRNA vaccines maintains over 70% protection against hospitalisation and death in breakthrough confirmed omicron infections.
Funding: Medical Research Council, UK Research and Innovation, Department of Health and Social Care, National Institute for Health Research, Community Jameel, and Engineering and Physical Sciences Research Council.
Copyright © 2022 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests GD declares that his employer UK Health Security Agency (previously operating as Public Health England) received funding from GlaxoSmithKline for a research project related to influenza antiviral treatment. This preceded and had no relation to COVID-19, and GD had no role in and received no funding from the project. All other authors declare no competing interests.
Figures
Comment in
-
Omicron: fewer adverse outcomes come with new dangers.Lancet. 2022 Apr 2;399(10332):1280-1281. doi: 10.1016/S0140-6736(22)00514-1. Epub 2022 Mar 16. Lancet. 2022. PMID: 35305298 Free PMC article. No abstract available.
-
Misclassification bias in estimating clinical severity of SARS-CoV-2 variants.Lancet. 2022 Sep 10;400(10355):809. doi: 10.1016/S0140-6736(22)01469-6. Lancet. 2022. PMID: 36088948 Free PMC article. No abstract available.
Similar articles
-
Protection against symptomatic infection with delta (B.1.617.2) and omicron (B.1.1.529) BA.1 and BA.2 SARS-CoV-2 variants after previous infection and vaccination in adolescents in England, August, 2021-March, 2022: a national, observational, test-negative, case-control study.Lancet Infect Dis. 2023 Apr;23(4):435-444. doi: 10.1016/S1473-3099(22)00729-0. Epub 2022 Nov 24. Lancet Infect Dis. 2023. PMID: 36436536 Free PMC article.
-
Hospital admission and emergency care attendance risk for SARS-CoV-2 delta (B.1.617.2) compared with alpha (B.1.1.7) variants of concern: a cohort study.Lancet Infect Dis. 2022 Jan;22(1):35-42. doi: 10.1016/S1473-3099(21)00475-8. Epub 2021 Aug 27. Lancet Infect Dis. 2022. PMID: 34461056 Free PMC article.
-
Effectiveness of primary series and booster vaccination against SARS-CoV-2 infection and hospitalisation among adolescents aged 12-17 years in Singapore: a national cohort study.Lancet Infect Dis. 2023 Feb;23(2):177-182. doi: 10.1016/S1473-3099(22)00573-4. Epub 2022 Sep 28. Lancet Infect Dis. 2023. PMID: 36182678 Free PMC article.
-
COVID-19 Vaccine Effectiveness of Booster Doses Against Delta and Omicron Variants Over Follow-up Times Using Longitudinal Meta-analysis.J Res Health Sci. 2024 Sep 30;24(4):e00626. doi: 10.34172/jrhs.2024.161. Epub 2024 Sep 30. J Res Health Sci. 2024. PMID: 39431651 Free PMC article.
-
Viral and antibody dynamics of acute infection with SARS-CoV-2 omicron variant (B.1.1.529): a prospective cohort study from Shenzhen, China.Lancet Microbe. 2023 Aug;4(8):e632-e641. doi: 10.1016/S2666-5247(23)00139-8. Epub 2023 Jul 14. Lancet Microbe. 2023. PMID: 37459867 Review.
Cited by
-
The SARS-CoV-2 Delta-Omicron Recombinant Lineage (XD) Exhibits Immune-Escape Properties Similar to the Omicron (BA.1) Variant.Int J Mol Sci. 2022 Nov 14;23(22):14057. doi: 10.3390/ijms232214057. Int J Mol Sci. 2022. PMID: 36430535 Free PMC article.
-
Comparative analysis of the safety and effectiveness of Nirmatrelvir-Ritonavir and Azvudine in older patients with COVID-19: a retrospective study from a tertiary hospital in China.Front Pharmacol. 2024 Jul 22;15:1362345. doi: 10.3389/fphar.2024.1362345. eCollection 2024. Front Pharmacol. 2024. PMID: 39104387 Free PMC article.
-
Variable detection of Omicron-BA.1 and -BA.2 by SARS-CoV-2 rapid antigen tests.Med Microbiol Immunol. 2023 Feb;212(1):13-23. doi: 10.1007/s00430-022-00752-7. Epub 2022 Nov 12. Med Microbiol Immunol. 2023. PMID: 36370197 Free PMC article.
-
Protection against symptomatic infection with delta (B.1.617.2) and omicron (B.1.1.529) BA.1 and BA.2 SARS-CoV-2 variants after previous infection and vaccination in adolescents in England, August, 2021-March, 2022: a national, observational, test-negative, case-control study.Lancet Infect Dis. 2023 Apr;23(4):435-444. doi: 10.1016/S1473-3099(22)00729-0. Epub 2022 Nov 24. Lancet Infect Dis. 2023. PMID: 36436536 Free PMC article.
-
Evaluation of the Neutralizing Antibody STE90-C11 against SARS-CoV-2 Delta Infection and Its Recognition of Other Variants of Concerns.Viruses. 2023 Oct 25;15(11):2153. doi: 10.3390/v15112153. Viruses. 2023. PMID: 38005829 Free PMC article.
References
-
- WHO Classification of omicron (B.1.1.529): SARS-CoV-2 variant of concern. Nov 26, 2021. https://www.who.int/news/item/26-11-2021-classification-of-omicron-(b.1....
-
- Centers for Disease Control and Prevention Science brief: omicron (B.1.1.529) variant. https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/scienti... - PubMed
-
- GISAID Tracking of variants. https://www.gisaid.org/hcov19-variants/
-
- Birrell P, Blake J, van Leeuwen E, MRC Biostatistics Unit COVID-19 Working Group. De Angelis D. Report on nowcasting and forecasting. Dec 9, 2021. https://www.mrc-bsu.cam.ac.uk/now-casting/report-on-nowcasting-and-forec... - PubMed
-
- UK Health Security Agency SARS-CoV-2 variants of concern and variants under investigation in England: technical briefing 35. 2022. https://assets.publishing.service.gov.uk/government/uploads/system/uploa...
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

