Covid-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant
- PMID: 35249272
- PMCID: PMC8908811
- DOI: 10.1056/NEJMoa2119451
Covid-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant
Abstract
Background: A rapid increase in coronavirus disease 2019 (Covid-19) cases due to the omicron (B.1.1.529) variant of severe acute respiratory syndrome coronavirus 2 in highly vaccinated populations has aroused concerns about the effectiveness of current vaccines.
Methods: We used a test-negative case-control design to estimate vaccine effectiveness against symptomatic disease caused by the omicron and delta (B.1.617.2) variants in England. Vaccine effectiveness was calculated after primary immunization with two doses of BNT162b2 (Pfizer-BioNTech), ChAdOx1 nCoV-19 (AstraZeneca), or mRNA-1273 (Moderna) vaccine and after a booster dose of BNT162b2, ChAdOx1 nCoV-19, or mRNA-1273.
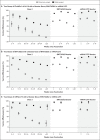
Results: Between November 27, 2021, and January 12, 2022, a total of 886,774 eligible persons infected with the omicron variant, 204,154 eligible persons infected with the delta variant, and 1,572,621 eligible test-negative controls were identified. At all time points investigated and for all combinations of primary course and booster vaccines, vaccine effectiveness against symptomatic disease was higher for the delta variant than for the omicron variant. No effect against the omicron variant was noted from 20 weeks after two ChAdOx1 nCoV-19 doses, whereas vaccine effectiveness after two BNT162b2 doses was 65.5% (95% confidence interval [CI], 63.9 to 67.0) at 2 to 4 weeks, dropping to 8.8% (95% CI, 7.0 to 10.5) at 25 or more weeks. Among ChAdOx1 nCoV-19 primary course recipients, vaccine effectiveness increased to 62.4% (95% CI, 61.8 to 63.0) at 2 to 4 weeks after a BNT162b2 booster before decreasing to 39.6% (95% CI, 38.0 to 41.1) at 10 or more weeks. Among BNT162b2 primary course recipients, vaccine effectiveness increased to 67.2% (95% CI, 66.5 to 67.8) at 2 to 4 weeks after a BNT162b2 booster before declining to 45.7% (95% CI, 44.7 to 46.7) at 10 or more weeks. Vaccine effectiveness after a ChAdOx1 nCoV-19 primary course increased to 70.1% (95% CI, 69.5 to 70.7) at 2 to 4 weeks after an mRNA-1273 booster and decreased to 60.9% (95% CI, 59.7 to 62.1) at 5 to 9 weeks. After a BNT162b2 primary course, the mRNA-1273 booster increased vaccine effectiveness to 73.9% (95% CI, 73.1 to 74.6) at 2 to 4 weeks; vaccine effectiveness fell to 64.4% (95% CI, 62.6 to 66.1) at 5 to 9 weeks.
Conclusions: Primary immunization with two doses of ChAdOx1 nCoV-19 or BNT162b2 vaccine provided limited protection against symptomatic disease caused by the omicron variant. A BNT162b2 or mRNA-1273 booster after either the ChAdOx1 nCoV-19 or BNT162b2 primary course substantially increased protection, but that protection waned over time. (Funded by the U.K. Health Security Agency.).
Copyright © 2022 Massachusetts Medical Society.
Figures
Comment in
-
Neutralization of SARS-CoV-2 Omicron BA.1, BA.4, and BA.5 by primary ChAdOx1 nCoV-19, mRNA-1273, MVC-COV1901 and booster mRNA-1273 vaccination.Infection. 2023 Apr;51(2):531-534. doi: 10.1007/s15010-022-01922-8. Epub 2022 Sep 15. Infection. 2023. PMID: 36109464 Free PMC article. No abstract available.
Similar articles
-
Effect of mRNA Vaccine Boosters against SARS-CoV-2 Omicron Infection in Qatar.N Engl J Med. 2022 May 12;386(19):1804-1816. doi: 10.1056/NEJMoa2200797. Epub 2022 Mar 9. N Engl J Med. 2022. PMID: 35263534 Free PMC article.
-
Comparative effectiveness of different primary vaccination courses on mRNA-based booster vaccines against SARs-COV-2 infections: a time-varying cohort analysis using trial emulation in the Virus Watch community cohort.Int J Epidemiol. 2023 Apr 19;52(2):342-354. doi: 10.1093/ije/dyad002. Int J Epidemiol. 2023. PMID: 36655537 Free PMC article.
-
Immunogenicity and reactogenicity against the SARS-CoV-2 variants following heterologous primary series involving CoronaVac, ChAdox1 nCov-19 and BNT162b2 plus BNT162b2 booster vaccination: An open-label randomized study in healthy Thai adults.Hum Vaccin Immunother. 2022 Nov 30;18(6):2091865. doi: 10.1080/21645515.2022.2091865. Epub 2022 Jul 11. Hum Vaccin Immunother. 2022. PMID: 35816053 Free PMC article. Clinical Trial.
-
The Omicron variant wave: Where are we now and what are the prospects?J Chin Med Assoc. 2023 Feb 1;86(2):135-137. doi: 10.1097/JCMA.0000000000000863. Epub 2022 Dec 13. J Chin Med Assoc. 2023. PMID: 36524941 Review.
-
Role of COVID-19 Vaccines in SARS-CoV-2 Variants.Front Immunol. 2022 May 20;13:898192. doi: 10.3389/fimmu.2022.898192. eCollection 2022. Front Immunol. 2022. PMID: 35669787 Free PMC article. Review.
Cited by
-
Description of Hospitalizations due to the Severe Acute Respiratory Syndrome Coronavirus 2 Omicron Variant Based on Vaccination Status.Open Forum Infect Dis. 2022 Aug 25;9(9):ofac438. doi: 10.1093/ofid/ofac438. eCollection 2022 Sep. Open Forum Infect Dis. 2022. PMID: 36092825 Free PMC article.
-
Designing nanotheranostics with machine learning.Nat Nanotechnol. 2024 Dec;19(12):1769-1781. doi: 10.1038/s41565-024-01753-8. Epub 2024 Oct 3. Nat Nanotechnol. 2024. PMID: 39362960 Review.
-
Adherence to stay-at-home orders: awareness, implementation and difficulties of officially ordered quarantine measures in the context of the COVID-19 pandemic in Cologne - a retrospective cohort study.BMJ Open. 2022 Nov 2;12(11):e063358. doi: 10.1136/bmjopen-2022-063358. BMJ Open. 2022. PMID: 36323466 Free PMC article.
-
Infection with SARS-CoV-2 following Second Dose Pfizer-BioNTech mRNA COVID-19 Vaccine BNT162b2 in Danish Adolescents Aged 12-18 Years: A Real-World Nationwide Danish Cohort Study.Viruses. 2023 Dec 29;16(1):56. doi: 10.3390/v16010056. Viruses. 2023. PMID: 38257757 Free PMC article.
-
Effectiveness of Bivalent mRNA Booster Vaccine Against COVID-19 in Korea.J Korean Med Sci. 2024 Jan 22;39(3):e15. doi: 10.3346/jkms.2024.39.e15. J Korean Med Sci. 2024. PMID: 38258360 Free PMC article.
References
-
- World Health Organization. Classification of omicron (B.1.1.529): SARS-CoV-2 variant of concern. November 26, 2021. (https://www.who.int/news/item/26-11-2021-classification-of-omicron-(b.1....).
-
- European Centre for Disease Prevention and Control. Implications of the further emergence and spread of the SARS-CoV-2 B.1.1.529 variant of concern (omicron) for the EU/EEA — first update. December 2, 2021. (https://www.ecdc.europa.eu/sites/default/files/documents/threat-assessme...).
-
- Cele S, Jackson L, Khoury DS, et al. SARS-CoV-2 omicron has extensive but incomplete escape of Pfizer BNT162b2 elicited neutralization and requires ACE2 for infection. December 17, 2021. (https://www.medrxiv.org/content/10.1101/2021.12.08.21267417v3). preprint.
-
- Wilhelm A, Widera M, Grikscheit K, et al. Reduced neutralization of SARS-CoV-2 omicron variant by vaccine sera and monoclonal antibodies. December 13, 2021. (https://www.medrxiv.org/content/10.1101/2021.12.07.21267432v4). preprint.
-
- Dejnirattisai W, Huo J, Zhou D, et al. Omicron-B.1.1.529 leads to widespread escape from neutralizing antibody responses. December 22, 2021. (https://www.biorxiv.org/content/10.1101/2021.12.03.471045v2). preprint. - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
