The Novel PKC Activator 10-Methyl-Aplog-1 Combined with JQ1 Induced Strong and Synergistic HIV Reactivation with Tolerable Global T Cell Activation
- PMID: 34696466
- PMCID: PMC8541327
- DOI: 10.3390/v13102037
The Novel PKC Activator 10-Methyl-Aplog-1 Combined with JQ1 Induced Strong and Synergistic HIV Reactivation with Tolerable Global T Cell Activation
Abstract
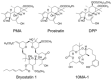
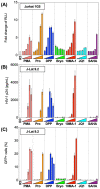
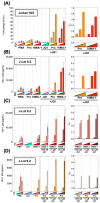
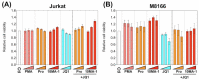
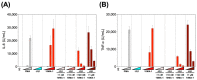
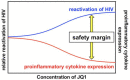
The presence of latent human immunodeficiency virus (HIV) reservoirs is a major obstacle to a cure. The "shock and kill" therapy is based on the concept that latent reservoirs in HIV carriers with antiretroviral therapy are reactivated by latency-reversing agents (LRAs), followed by elimination due to HIV-associated cell death or killing by virus-specific cytotoxic T lymphocytes. Protein kinase C (PKC) activators are considered robust LRAs as they efficiently reactivate latently infected HIV. However, various adverse events hamper the intervention trial of PKC activators as LRAs. We found in this study that a novel PKC activator, 10-Methyl-aplog-1 (10MA-1), combined with an inhibitor of bromodomain and extra-terminal domain motifs, JQ1, strongly and synergistically reactivated latently infected HIV. Notably, higher concentrations of 10MA-1 alone induced the predominant side effect, i.e., global T cell activation as defined by CD25 expression and pro-inflammatory cytokine production in primary CD4+ T lymphocytes; however, JQ1 efficiently suppressed the 10MA-1-induced side effect in a dose-dependent manner. Considering the reasonable accessibility and availability of 10MA-1 since the chemical synthesis of 10MA-1 requires fewer processes than that of bryostatin 1 or prostratin, our results suggest that the combination of 10MA-1 with JQ1 may be a promising pair of LRAs for the clinical application of the "shock and kill" therapy.
Keywords: 10-methyl-aplog-1; HIV; PKC activator; latency-reversing agents; shock and kill.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
4-Deoxyphorbol inhibits HIV-1 infection in synergism with antiretroviral drugs and reactivates viral reservoirs through PKC/MEK activation synergizing with vorinostat.Biochem Pharmacol. 2020 Jul;177:113937. doi: 10.1016/j.bcp.2020.113937. Epub 2020 Mar 26. Biochem Pharmacol. 2020. PMID: 32224142
-
An In-Depth Comparison of Latency-Reversing Agent Combinations in Various In Vitro and Ex Vivo HIV-1 Latency Models Identified Bryostatin-1+JQ1 and Ingenol-B+JQ1 to Potently Reactivate Viral Gene Expression.PLoS Pathog. 2015 Jul 30;11(7):e1005063. doi: 10.1371/journal.ppat.1005063. eCollection 2015 Jul. PLoS Pathog. 2015. PMID: 26225566 Free PMC article.
-
Alternate NF-κB-Independent Signaling Reactivation of Latent HIV-1 Provirus.J Virol. 2019 Aug 28;93(18):e00495-19. doi: 10.1128/JVI.00495-19. Print 2019 Sep 15. J Virol. 2019. PMID: 31243131 Free PMC article.
-
New Latency Reversing Agents for HIV-1 Cure: Insights from Nonhuman Primate Models.Viruses. 2021 Aug 6;13(8):1560. doi: 10.3390/v13081560. Viruses. 2021. PMID: 34452425 Free PMC article. Review.
-
Getting the "Kill" into "Shock and Kill": Strategies to Eliminate Latent HIV.Cell Host Microbe. 2018 Jan 10;23(1):14-26. doi: 10.1016/j.chom.2017.12.004. Cell Host Microbe. 2018. PMID: 29324227 Free PMC article. Review.
Cited by
-
Breaking the Silence: Regulation of HIV Transcription and Latency on the Road to a Cure.Viruses. 2023 Dec 15;15(12):2435. doi: 10.3390/v15122435. Viruses. 2023. PMID: 38140676 Free PMC article. Review.
-
The ingenol-based protein kinase C agonist GSK445A is a potent inducer of HIV and SIV RNA transcription.PLoS Pathog. 2022 Jan 18;18(1):e1010245. doi: 10.1371/journal.ppat.1010245. eCollection 2022 Jan. PLoS Pathog. 2022. PMID: 35041707 Free PMC article.
-
Histone deacetylase inhibition reduces deleterious cytokine release induced by ingenol stimulation.Biochem Pharmacol. 2022 Jan;195:114844. doi: 10.1016/j.bcp.2021.114844. Epub 2021 Nov 18. Biochem Pharmacol. 2022. PMID: 34801521 Free PMC article.
-
Role of TLRs in HIV-1 Infection and Potential of TLR Agonists in HIV-1 Vaccine Development and Treatment Strategies.Pathogens. 2023 Jan 5;12(1):92. doi: 10.3390/pathogens12010092. Pathogens. 2023. PMID: 36678440 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

