Inhibition of Glycine Re-Uptake: A Potential Approach for Treating Pain by Augmenting Glycine-Mediated Spinal Neurotransmission and Blunting Central Nociceptive Signaling
- PMID: 34200954
- PMCID: PMC8230656
- DOI: 10.3390/biom11060864
Inhibition of Glycine Re-Uptake: A Potential Approach for Treating Pain by Augmenting Glycine-Mediated Spinal Neurotransmission and Blunting Central Nociceptive Signaling
Abstract
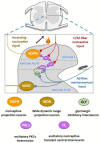
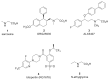
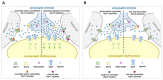
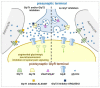
Among the myriad of cellular and molecular processes identified as contributing to pathological pain, disinhibition of spinal cord nociceptive signaling to higher cortical centers plays a critical role. Importantly, evidence suggests that impaired glycinergic neurotransmission develops in the dorsal horn of the spinal cord in inflammatory and neuropathic pain models and is a key maladaptive mechanism causing mechanical hyperalgesia and allodynia. Thus, it has been hypothesized that pharmacological agents capable of augmenting glycinergic tone within the dorsal horn may be able to blunt or block aberrant nociceptor signaling to the brain and serve as a novel class of analgesics for various pathological pain states. Indeed, drugs that enhance dysfunctional glycinergic transmission, and in particular inhibitors of the glycine transporters (GlyT1 and GlyT2), are generating widespread interest as a potential class of novel analgesics. The GlyTs are Na+/Cl--dependent transporters of the solute carrier 6 (SLC6) family and it has been proposed that the inhibition of them presents a possible mechanism by which to increase spinal extracellular glycine concentrations and enhance GlyR-mediated inhibitory neurotransmission in the dorsal horn. Various inhibitors of both GlyT1 and GlyT2 have demonstrated broad analgesic efficacy in several preclinical models of acute and chronic pain, providing promise for the approach to deliver a first-in-class non-opioid analgesic with a mechanism of action differentiated from current standard of care. This review will highlight the therapeutic potential of GlyT inhibitors as a novel class of analgesics, present recent advances reported for the field, and discuss the key challenges associated with the development of a GlyT inhibitor into a safe and effective agent to treat pain.
Keywords: GABA; N-methyl-d-aspartate (NMDA) receptor; allodynia; dorsal horn; glycine; glycine receptor (GlyR); glycine transporter (GlyT); hyperalgesia; inflammatory pain; neuropathic pain; nociceptor; spinal cord.
Conflict of interest statement
The author declares no conflict of interest.
Figures
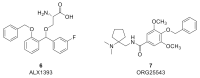

Comment in
-
Glycine Transporters and Receptors as Targets for Analgesics.Biomolecules. 2021 Nov 11;11(11):1676. doi: 10.3390/biom11111676. Biomolecules. 2021. PMID: 34827674 Free PMC article.
Similar articles
-
Glycine transporter GlyT1, but not GlyT2, is expressed in rat dorsal root ganglion--Possible implications for neuropathic pain.Neurosci Lett. 2015 Jul 23;600:213-9. doi: 10.1016/j.neulet.2015.06.026. Epub 2015 Jun 20. Neurosci Lett. 2015. PMID: 26101830
-
Glycine transporter inhibitors: A new avenue for managing neuropathic pain.Brain Res Bull. 2019 Oct;152:143-158. doi: 10.1016/j.brainresbull.2019.07.008. Epub 2019 Jul 11. Brain Res Bull. 2019. PMID: 31302238 Review.
-
Activity of novel lipid glycine transporter inhibitors on synaptic signalling in the dorsal horn of the spinal cord.Br J Pharmacol. 2018 Jun;175(12):2337-2347. doi: 10.1111/bph.14189. Epub 2018 Apr 14. Br J Pharmacol. 2018. PMID: 29500820 Free PMC article.
-
Glycine transporter inhibitors as a novel drug discovery strategy for neuropathic pain.Pharmacol Ther. 2009 Jul;123(1):54-79. doi: 10.1016/j.pharmthera.2009.03.018. Epub 2009 Apr 23. Pharmacol Ther. 2009. PMID: 19393690
-
Neurobiology of glycine transporters: From molecules to behavior.Neurosci Biobehav Rev. 2020 Nov;118:97-110. doi: 10.1016/j.neubiorev.2020.07.025. Epub 2020 Jul 24. Neurosci Biobehav Rev. 2020. PMID: 32712279 Review.
Cited by
-
Analysis of Binding Determinants for Different Classes of Competitive and Noncompetitive Inhibitors of Glycine Transporters.Int J Mol Sci. 2022 Jul 21;23(14):8050. doi: 10.3390/ijms23148050. Int J Mol Sci. 2022. PMID: 35887394 Free PMC article.
-
The GlyT1-inhibitor Org 24598 facilitates the alcohol deprivation abolishing and dopamine elevating effects of bupropion + varenicline in rats.J Neural Transm (Vienna). 2024 Jan;131(1):95-106. doi: 10.1007/s00702-023-02701-x. Epub 2023 Sep 29. J Neural Transm (Vienna). 2024. PMID: 37773223 Free PMC article.
-
Glycinergic Modulation of Pain in Behavioral Animal Models.Front Pharmacol. 2022 May 25;13:860903. doi: 10.3389/fphar.2022.860903. eCollection 2022. Front Pharmacol. 2022. PMID: 35694265 Free PMC article. Review.
-
Disinhibition Is an Essential Network Motif Coordinated by GABA Levels and GABA B Receptors.Int J Mol Sci. 2024 Jan 22;25(2):1340. doi: 10.3390/ijms25021340. Int J Mol Sci. 2024. PMID: 38279339 Free PMC article. Review.
-
Glycine Transporter 1 Inhibitors: Predictions on Their Possible Mechanisms in the Development of Opioid Analgesic Tolerance.Biomedicines. 2024 Feb 12;12(2):421. doi: 10.3390/biomedicines12020421. Biomedicines. 2024. PMID: 38398023 Free PMC article. Review.
References
-
- Zeilhofer H.U. Spinal neuroplasticity in chronic pain. e-Neuroforum. 2011;2:35–41. doi: 10.1007/s13295-011-0018-1. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

