Designing a SARS-CoV-2 T-Cell-Inducing Vaccine for High-Risk Patient Groups
- PMID: 33923363
- PMCID: PMC8146137
- DOI: 10.3390/vaccines9050428
Designing a SARS-CoV-2 T-Cell-Inducing Vaccine for High-Risk Patient Groups
Abstract
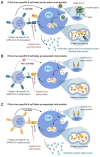
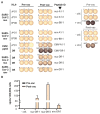
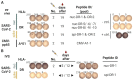
We describe the results of two vaccinations of a self-experimenting healthy volunteer with SARS-CoV-2-derived peptides performed in March and April 2020, respectively. The first set of peptides contained eight peptides predicted to bind to the individual's HLA molecules. The second set consisted of ten peptides predicted to bind promiscuously to several HLA-DR allotypes. The vaccine formulation contained the new TLR 1/2 agonist XS15 and was administered as an emulsion in Montanide as a single subcutaneous injection. Peripheral blood mononuclear cells isolated from blood drawn before and after vaccinations were assessed using Interferon-γ ELISpot assays and intracellular cytokine staining. We detected vaccine-induced CD4 T cell responses against six out of 11 peptides predicted to bind to HLA-DR after 19 days, following vaccination, for one peptide already at day 12. We used these results to support the design of a T-cell-inducing vaccine for application in high-risk patients, with weakened lymphocyte performance. Meanwhile, an according vaccine, incorporating T cell epitopes predominant in convalescents, is undergoing clinical trial testing.
Keywords: COVID-19; SARS-CoV-2; adjuvant; high-risk patient; lipopeptide; peptide vaccine.
Conflict of interest statement
H.G. Rammensee has ownership interest in Immatics Biotechnologies GmbH, CureVac AG, Bamomab GmbH, and Synimmune GmbH. H.G. Rammensee and K.H. Wiesmüller share the patent for XS15. K.H. Wiesmüller further holds ownership interest in EMC microcollections GmbH. O. Planz has ownership interest in Atriva Therapeutics GmbH and is a consultant for Atriva Therapeutics GmbH. H. Hoffmann is an employee of Atriva Therapeutics GmbH. H.G. Rammensee, A. Nelde, J.S. Walz, S.P. Haen, and M.W. Löffler are the inventors of patents for vaccine peptides owned by Immatics. H.G. Rammensee, A. Nelde, and J.S. Walz hold patents on peptides described in this manuscript secured under the numbers 20_169_047.6 and 20_190_070.1. M.W. Löffler acts as a paid consultant in cancer immunology for Boehringer Ingelheim Pharma GmbH & Co. KG. G. Tabatabai reports personal fees (advisory board, speaker’s fees) from AbbVie, Bayer, Bristol-Myers-Squibb, Medac, Novocure, travel grants from Bristol-Myers-Squibb, educational and travel grants from Novocure, research grants from Roche Diagnostics, and research and travel grants from Medac. All other authors declare no potential conflicts of interest.
Figures
Similar articles
-
A new synthetic toll-like receptor 1/2 ligand is an efficient adjuvant for peptide vaccination in a human volunteer.J Immunother Cancer. 2019 Nov 15;7(1):307. doi: 10.1186/s40425-019-0796-5. J Immunother Cancer. 2019. PMID: 31730025 Free PMC article.
-
SARS-CoV-2 spike protein-derived immunogenic peptides that are promiscuously presented by several HLA-class II molecules and their potential for inducing acquired immunity.Heliyon. 2023 Sep 20;9(9):e20192. doi: 10.1016/j.heliyon.2023.e20192. eCollection 2023 Sep. Heliyon. 2023. PMID: 37809871 Free PMC article.
-
Immunization of patients with melanoma peptide vaccines: immunologic assessment using the ELISPOT assay.Cancer J Sci Am. 1998 Sep-Oct;4(5):316-23. Cancer J Sci Am. 1998. PMID: 9815296 Clinical Trial.
-
Identification of HLA-A*02:01-restricted candidate epitopes derived from the non-structural polyprotein 1a of SARS-CoV-2 that may be natural targets of CD8+ T cell recognition in vivo.J Virol. 2021 Mar 1;95(5):e01837-20. doi: 10.1128/JVI.01837-20. Epub 2020 Dec 2. J Virol. 2021. PMID: 33268522 Free PMC article.
-
Immune modulations during chemoimmunotherapy & novel vaccine strategies--in metastatic melanoma and non small-cell lung cancer.Dan Med J. 2013 Dec;60(12):B4774. Dan Med J. 2013. PMID: 24355457 Review.
Cited by
-
Simultaneous Identification of Functional Antigen-Specific CD8+ and CD4+ Cells after In Vitro Expansion Using Elongated Peptides.Cells. 2022 Oct 31;11(21):3451. doi: 10.3390/cells11213451. Cells. 2022. PMID: 36359847 Free PMC article.
-
AI Aided Design of Epitope-Based Vaccine for the Induction of Cellular Immune Responses Against SARS-CoV-2.Front Genet. 2021 Mar 25;12:602196. doi: 10.3389/fgene.2021.602196. eCollection 2021. Front Genet. 2021. PMID: 33841493 Free PMC article.
-
A systemic review of T-cell epitopes defined from the proteome of SARS-CoV-2.Virus Res. 2023 Jan 15;324:199024. doi: 10.1016/j.virusres.2022.199024. Epub 2022 Dec 13. Virus Res. 2023. PMID: 36526016 Free PMC article.
-
Recent Advances in the Development of Toll-like Receptor Agonist-Based Vaccine Adjuvants for Infectious Diseases.Pharmaceutics. 2022 Feb 16;14(2):423. doi: 10.3390/pharmaceutics14020423. Pharmaceutics. 2022. PMID: 35214155 Free PMC article. Review.
-
A COVID-19 peptide vaccine for the induction of SARS-CoV-2 T cell immunity.Nature. 2022 Jan;601(7894):617-622. doi: 10.1038/s41586-021-04232-5. Epub 2021 Nov 23. Nature. 2022. PMID: 34814158 Free PMC article. Clinical Trial.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

