RIPK3-mediated inflammation is a conserved β cell response to ER stress
- PMID: 33355143
- PMCID: PMC11206196
- DOI: 10.1126/sciadv.abd7272
RIPK3-mediated inflammation is a conserved β cell response to ER stress
Abstract
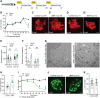
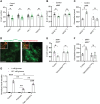
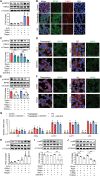
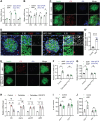
Islet inflammation is an important etiopathology of type 2 diabetes; however, the underlying mechanisms are not well defined. Using complementary experimental models, we discovered RIPK3-dependent IL1B induction in β cells as an instigator of islet inflammation. In cultured β cells, ER stress activated RIPK3, leading to NF-kB-mediated proinflammatory gene expression. In a zebrafish muscle insulin resistance model, overnutrition caused islet inflammation, β cell dysfunction, and loss in an ER stress-, ripk3-, and il1b-dependent manner. In mouse islets, high-fat diet triggered the IL1B expression in β cells before macrophage recruitment in vivo, and RIPK3 inhibition suppressed palmitate-induced β cell dysfunction and Il1b expression in vitro. Furthermore, in human islets grafted in hyperglycemic mice, a marked increase in ER stress, RIPK3, and NF-kB activation in β cells were accompanied with murine macrophage infiltration. Thus, RIPK3-mediated induction of proinflammatory mediators is a conserved, previously unrecognized β cell response to metabolic stress and a mediator of the ensuing islet inflammation.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures
Similar articles
-
Macrophages and neutrophils are necessary for ER stress-induced β cell loss.Cell Rep. 2022 Aug 23;40(8):111255. doi: 10.1016/j.celrep.2022.111255. Cell Rep. 2022. PMID: 36001973 Free PMC article.
-
Macrophage RIPK3 triggers inflammation and cell death via the XBP1-Foxo1 axis in liver ischaemia-reperfusion injury.JHEP Rep. 2023 Aug 12;5(11):100879. doi: 10.1016/j.jhepr.2023.100879. eCollection 2023 Nov. JHEP Rep. 2023. PMID: 37841640 Free PMC article.
-
Macrophage Notch1 inhibits TAK1 function and RIPK3-mediated hepatocyte necroptosis through activation of β-catenin signaling in liver ischemia and reperfusion injury.Cell Commun Signal. 2022 Sep 16;20(1):144. doi: 10.1186/s12964-022-00901-8. Cell Commun Signal. 2022. PMID: 36114543 Free PMC article.
-
RIPK1 and RIPK3 regulate TNFα-induced β-cell death in concert with caspase activity.Mol Metab. 2022 Nov;65:101582. doi: 10.1016/j.molmet.2022.101582. Epub 2022 Aug 24. Mol Metab. 2022. PMID: 36030035 Free PMC article.
-
Signaling between pancreatic β cells and macrophages via S100 calcium-binding protein A8 exacerbates β-cell apoptosis and islet inflammation.J Biol Chem. 2018 Apr 20;293(16):5934-5946. doi: 10.1074/jbc.M117.809228. Epub 2018 Mar 1. J Biol Chem. 2018. PMID: 29496993 Free PMC article.
Cited by
-
Type 2 diabetes mellitus in adults: pathogenesis, prevention and therapy.Signal Transduct Target Ther. 2024 Oct 2;9(1):262. doi: 10.1038/s41392-024-01951-9. Signal Transduct Target Ther. 2024. PMID: 39353925 Free PMC article. Review.
-
RISING STARS: Evidence for established and emerging forms of β-cell death.J Endocrinol. 2024 Jul 4;262(2):e230378. doi: 10.1530/JOE-23-0378. Print 2024 Aug 1. J Endocrinol. 2024. PMID: 38842911 Free PMC article. Review.
-
RIPK3 signaling and its role in regulated cell death and diseases.Cell Death Discov. 2024 Apr 29;10(1):200. doi: 10.1038/s41420-024-01957-w. Cell Death Discov. 2024. PMID: 38684668 Free PMC article. Review.
-
Animal Models for Understanding the Mechanisms of Beta Cell Death during Type 2 Diabetes Pathogenesis.Biomedicines. 2024 Feb 20;12(3):473. doi: 10.3390/biomedicines12030473. Biomedicines. 2024. PMID: 38540087 Free PMC article. Review.
-
Inflammation in Development and Aging: Insights from the Zebrafish Model.Int J Mol Sci. 2024 Feb 10;25(4):2145. doi: 10.3390/ijms25042145. Int J Mol Sci. 2024. PMID: 38396822 Free PMC article. Review.
References
-
- Cho N. H., Shaw J. E., Karuranga S., Huang Y., da Rocha Fernandes J. D., Ohlrogge A. W., Malanda B., IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 138, 271–281 (2018). - PubMed
-
- Lytrivi M., Igoillo-Esteve M., Cnop M., Inflammatory stress in islet β-cells: Therapeutic implications for type 2 diabetes? Curr. Opin. Pharmacol. 43, 40–45 (2018). - PubMed
-
- Ying W., Lee Y. S., Dong Y., Seidman J. S., Yang M., Isaac R., Seo J. B., Yang B.-H., Wollam J., Riopel M., McNelis J., Glass C. K., Olefsky J. M., Fu W., Expansion of Islet-resident macrophages leads to inflammation affecting β cell proliferation and function in obesity. Cell Metab. 29, 457–474.e5 (2019). - PMC - PubMed
Publication types
Grants and funding
- R01 DK097392/DK/NIDDK NIH HHS/United States
- UC4 DK104211/DK/NIDDK NIH HHS/United States
- DK104211/DK/NIDDK NIH HHS/United States
- R01 DK115620/DK/NIDDK NIH HHS/United States
- UC4 DK112232/DK/NIDDK NIH HHS/United States
- DK89572/DK/NIDDK NIH HHS/United States
- R24 DK106755/DK/NIDDK NIH HHS/United States
- U54 EY032442/EY/NEI NIH HHS/United States
- R01 DK117147/DK/NIDDK NIH HHS/United States
- DK108120/DK/NIDDK NIH HHS/United States
- DK106755/DK/NIDDK NIH HHS/United States
- UC4 DK098085/DK/NIDDK NIH HHS/United States
- UC4 DK108120/DK/NIDDK NIH HHS/United States
- S10 OD021630/OD/NIH HHS/United States
- U24 DK098085/DK/NIDDK NIH HHS/United States
- R01 DK109407/DK/NIDDK NIH HHS/United States
- R01 HL098085/HL/NHLBI NIH HHS/United States
- U01 DK072473/DK/NIDDK NIH HHS/United States
- DK109407/DK/NIDDK NIH HHS/United States
- U01 DK089572/DK/NIDDK NIH HHS/United States
- DK112232/DK/NIDDK NIH HHS/United States
- I01 BX000666/BX/BLRD VA/United States
- P30 DK020593/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

