Helicobacter pylori Infection Impairs Endothelial Function Through an Exosome-Mediated Mechanism
- PMID: 32174233
- PMCID: PMC7335532
- DOI: 10.1161/JAHA.119.014120
Helicobacter pylori Infection Impairs Endothelial Function Through an Exosome-Mediated Mechanism
Abstract
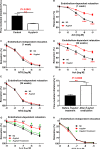
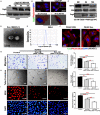
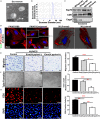
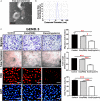
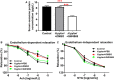
Background Epidemiological studies have suggested an association between Helicobacter pylori (H pylori) infection and atherosclerosis through undefined mechanisms. Endothelial dysfunction is critical to the development of atherosclerosis and related cardiovascular diseases. The present study was designed to test the hypothesis that H pylori infection impaires endothelial function through exosome-mediated mechanisms. Methods and Results Young male and female patients (18-35 years old) with and without H pylori infection were recruited to minimize the chance of potential risk factors for endothelial dysfunction for the study. Endothelium-dependent flow-mediated vasodilatation of the brachial artery was evaluated in the patients and control subjects. Mouse infection models with CagA+H pylori from a gastric ulcer patient were created to determine if H pylori infection-induced endothelial dysfunction could be reproduced in animal models. H pylori infection significantly decreased endothelium-dependent flow-mediated vasodilatation in young patients and significantly attenuated acetylcholine-induced endothelium-dependent aortic relaxation without change in nitroglycerin-induced endothelium-independent vascular relaxation in mice. H pylori eradication significantly improved endothelium-dependent vasodilation in both patients and mice with H pylori infection. Exosomes from conditioned media of human gastric epithelial cells cultured with CagA+H pylori or serum exosomes from patients and mice with H pylori infection significantly decreased endothelial functions with decreased migration, tube formation, and proliferation in vitro. Inhibition of exosome secretion with GW4869 effectively preserved endothelial function in mice with H pylori infection. Conclusions H pylori infection impaired endothelial function in patients and mice through exosome-medicated mechanisms. The findings indicated that H pylori infection might be a novel risk factor for cardiovascular diseases.
Keywords: Helicobacter pylori; cardiovascular risk factor; endothelial dysfunction; exosomes.
Figures
Similar articles
-
CagA+ Helicobacter pylori, Not CagA- Helicobacter pylori, Infection Impairs Endothelial Function Through Exosomes-Mediated ROS Formation.Front Cardiovasc Med. 2022 Mar 31;9:881372. doi: 10.3389/fcvm.2022.881372. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 35433874 Free PMC article.
-
Helicobacter pylori infection promotes liver injury through an exosome-mediated mechanism.Microb Pathog. 2024 Oct;195:106898. doi: 10.1016/j.micpath.2024.106898. Epub 2024 Aug 28. Microb Pathog. 2024. PMID: 39208956
-
Helicobacter pylori infection selectively attenuates endothelial function in male mice via exosomes-mediated ROS production.Front Cell Infect Microbiol. 2023 May 18;13:1142387. doi: 10.3389/fcimb.2023.1142387. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37274312 Free PMC article.
-
[Advances in CagA protein and CagAmediated pathogenesis of Helicobacter pylori-A review].Wei Sheng Wu Xue Bao. 2016 Dec 4;56(12):1821-30. Wei Sheng Wu Xue Bao. 2016. PMID: 29741846 Review. Chinese.
-
Helicobacter pylori and apoptosis.Yale J Biol Med. 1998 Mar-Apr;71(2):53-61. Yale J Biol Med. 1998. PMID: 10378350 Free PMC article. Review.
Cited by
-
Exosomal CagA from Helicobacter pylori aggravates intestinal epithelium barrier dysfunction in chronic colitis by facilitating Claudin-2 expression.Gut Pathog. 2022 Mar 24;14(1):13. doi: 10.1186/s13099-022-00486-0. Gut Pathog. 2022. PMID: 35331316 Free PMC article.
-
CagA+ Helicobacter pylori, Not CagA- Helicobacter pylori, Infection Impairs Endothelial Function Through Exosomes-Mediated ROS Formation.Front Cardiovasc Med. 2022 Mar 31;9:881372. doi: 10.3389/fcvm.2022.881372. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 35433874 Free PMC article.
-
Inflammation at the crossroads of Helicobacter pylori and COVID-19.Future Microbiol. 2022 Jan;17(2):77-80. doi: 10.2217/fmb-2021-0250. Epub 2021 Dec 17. Future Microbiol. 2022. PMID: 34915742 Free PMC article. No abstract available.
-
Exosomes as New Biomarkers and Drug Delivery Tools for the Prevention and Treatment of Various Diseases: Current Perspectives.Int J Mol Sci. 2021 Jul 21;22(15):7763. doi: 10.3390/ijms22157763. Int J Mol Sci. 2021. PMID: 34360530 Free PMC article. Review.
-
Long-term Helicobacter pylori infection is associated with an increased risk of carotid plaque formation: a retrospective cohort study.Front Cardiovasc Med. 2024 Oct 24;11:1476435. doi: 10.3389/fcvm.2024.1476435. eCollection 2024. Front Cardiovasc Med. 2024. PMID: 39512368 Free PMC article.
References
-
- Mentis A, Lehours P, Megraud F. Epidemiology and diagnosis of Helicobacter pylori infection. Helicobacter. 2015;20(suppl 1):1–7. - PubMed
-
- Eusebi LH, Zagari RM, Bazzoli F. Epidemiology of Helicobacter pylori infection. Helicobacter. 2014;19(suppl 1):1–5. - PubMed
-
- Xu Z, Li J, Wang H, Xu G. Helicobacter pylori infection and atherosclerosis: is there a causal relationship? Eur J Clin Microbiol Infect Dis. 2017;36:2293–2301. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

