A new synthetic toll-like receptor 1/2 ligand is an efficient adjuvant for peptide vaccination in a human volunteer
- PMID: 31730025
- PMCID: PMC6858783
- DOI: 10.1186/s40425-019-0796-5
A new synthetic toll-like receptor 1/2 ligand is an efficient adjuvant for peptide vaccination in a human volunteer
Erratum in
-
Correction: A new synthetic toll-like receptor 1/2 ligand is an efficient adjuvant for peptide vaccination in a human volunteer.J Immunother Cancer. 2020 May;8(1):e0796-5corr1. doi: 10.1136/jitc-2020-0796-5corr1. J Immunother Cancer. 2020. PMID: 32414863 Free PMC article. No abstract available.
Abstract
Background: We previously showed that the bacterial lipopeptide Pam3Cys-Ser-Ser, meanwhile established as a toll-like receptor (TLR) 1/2 ligand, acts as a strong adjuvant for the induction of virus specific CD8+ T cells in mice, when covalently coupled to a synthetic peptide.
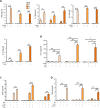
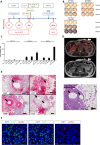
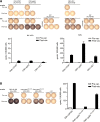
Case presentation: We now designed a new water-soluble synthetic Pam3Cys-derivative, named XS15 and characterized it in vitro by a TLR2 NF-κB luciferase reporter assay. Further, the capacity of XS15 to activate immune cells and stimulate peptide-specific CD8+ T and NK cells by 6-sulfo LacNAc+ monocytes was assessed by flow cytometry as well as cytokine induction using immunoassays. The induction of a functional immune response after vaccination of a volunteer with viral peptides was assessed by ELISpot assay and flow cytometry in peripheral blood cells and infiltrating cells at the vaccination site, as well as by immunohistochemistry and imaging. XS15 induced strong ex vivo CD8+ and TH1 CD4+ responses in a human volunteer upon a single injection of XS15 mixed to uncoupled peptides in a water-in-oil emulsion (Montanide™ ISA51 VG). A granuloma formed locally at the injection site containing highly activated functional CD4+ and CD8+ effector memory T cells. The total number of vaccine peptide-specific functional T cells was experimentally assessed and estimated to be 3.0 × 105 in the granuloma and 20.5 × 106 in peripheral blood.
Conclusion: Thus, in one volunteer we show a granuloma forming by peptides combined with an efficient adjuvant in a water-in-oil-emulsion, inducing antigen specific T cells detectable in circulation and at the vaccination site, after one single vaccination only. The ex vivo T cell responses in peripheral blood were detectable for more than one year and could be strongly boosted by a second vaccination. Hence, XS15 is a promising adjuvant candidate for peptide vaccination, in particular for tumor peptide vaccines in a personalized setting.
Keywords: Adjuvant; Immunotherapy; Lipopeptide; TLR1/2 ligand; Vaccines.
Conflict of interest statement
H.G. Rammensee has ownership interest (including patents) in Immatics Biotechnologies GmbH, CureVac AG, and Synimmune GmbH, further he shares the patent for XS15.
M.W. Löffler, D.J. Kowalewski, H. Schuster, S. Stevanović, and S.P. Haen are the inventors of patents for vaccine peptides owned by Immatics. D.J. Kowalewski, L. Backert, and H. Schuster are currently employees of Immatics Biotechnologies. P. Anoop Chandran is an employee of Adaptimmune Therapeutics Ltd. H. Zelba is employed by CeGaT GmbH.
K.H. Wiesmüller shares the patent for XS15 and holds ownership interest in EMC microcollections GmbH.
No competing interests were disclosed by the other authors.
Figures


Similar articles
-
Design of TLR2-ligand-synthetic long peptide conjugates for therapeutic vaccination of chronic HBV patients.Antiviral Res. 2020 Jun;178:104746. doi: 10.1016/j.antiviral.2020.104746. Epub 2020 Feb 17. Antiviral Res. 2020. PMID: 32081741
-
Efficient induction of antitumor immunity by synthetic toll-like receptor ligand-peptide conjugates.Cancer Immunol Res. 2014 Aug;2(8):756-64. doi: 10.1158/2326-6066.CIR-13-0223. Epub 2014 Apr 21. Cancer Immunol Res. 2014. PMID: 24950688
-
Vaccination with synthetic long peptide formulated with CpG in an oil-in-water emulsion induces robust E7-specific CD8 T cell responses and TC-1 tumor eradication.BMC Cancer. 2019 Jun 6;19(1):540. doi: 10.1186/s12885-019-5725-y. BMC Cancer. 2019. PMID: 31170937 Free PMC article.
-
Peptide emulsions in incomplete Freund's adjuvant create effective nurseries promoting egress of systemic CD4+ and CD8+ T cells for immunotherapy of cancer.J Immunother Cancer. 2022 Sep;10(9):e004709. doi: 10.1136/jitc-2022-004709. J Immunother Cancer. 2022. PMID: 36939214 Free PMC article. Review.
-
Dendritic cells, interleukin 12, and CD4+ lymphocytes in the initiation of class I-restricted reactivity to a tumor/self peptide.Crit Rev Immunol. 1998;18(1-2):87-98. doi: 10.1615/critrevimmunol.v18.i1-2.100. Crit Rev Immunol. 1998. PMID: 9419451 Review.
Cited by
-
Will Peptides Help to Stop COVID-19?Biochemistry (Mosc). 2022 Jul;87(7):590-604. doi: 10.1134/S0006297922070021. Biochemistry (Mosc). 2022. PMID: 36154880 Free PMC article. Review.
-
Cellular Immune Response after Vaccination in Patients with Cancer-Review on Past and Present Experiences.Vaccines (Basel). 2022 Jan 25;10(2):182. doi: 10.3390/vaccines10020182. Vaccines (Basel). 2022. PMID: 35214642 Free PMC article. Review.
-
Novel Synthetic Lipopeptides as Potential Mucosal Adjuvants Enhanced SARS-CoV-2 rRBD-Induced Immune Response.Front Immunol. 2022 Mar 9;13:833418. doi: 10.3389/fimmu.2022.833418. eCollection 2022. Front Immunol. 2022. PMID: 35356002 Free PMC article.
-
Cell-penetrating peptides enhance peptide vaccine accumulation and persistence in lymph nodes to drive immunogenicity.Proc Natl Acad Sci U S A. 2022 Aug 9;119(32):e2204078119. doi: 10.1073/pnas.2204078119. Epub 2022 Aug 1. Proc Natl Acad Sci U S A. 2022. PMID: 35914154 Free PMC article.
-
Designing a SARS-CoV-2 T-Cell-Inducing Vaccine for High-Risk Patient Groups.Vaccines (Basel). 2021 Apr 24;9(5):428. doi: 10.3390/vaccines9050428. Vaccines (Basel). 2021. PMID: 33923363 Free PMC article.
References
-
- Rini BI, Stenzl A, Zdrojowy R, et al. IMA901, a multipeptide cancer vaccine, plus sunitinib versus sunitinib alone, as first-line therapy for advanced or metastatic renal cell carcinoma (IMPRINT): a multicentre, open-label, randomised, controlled, phase 3 trial. Lancet Oncol. 2016;17(11):1599–1611. doi: 10.1016/S1470-2045(16)30408-9. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
