Ado-trastuzumab emtansine (T-DM1) in patients with HER2-amplified tumors excluding breast and gastric/gastroesophageal junction (GEJ) adenocarcinomas: results from the NCI-MATCH trial (EAY131) subprotocol Q
- PMID: 31504139
- PMCID: PMC6927318
- DOI: 10.1093/annonc/mdz291
Ado-trastuzumab emtansine (T-DM1) in patients with HER2-amplified tumors excluding breast and gastric/gastroesophageal junction (GEJ) adenocarcinomas: results from the NCI-MATCH trial (EAY131) subprotocol Q
Erratum in
-
Corrigendum to 'Ado-trastuzumab emtansine (T-DM1) in patients with HER2-amplified tumors excluding breast and gastric/gastroesophageal junction (GEJ) adenocarcinomas: results from the NCI-MATCH trial (EAY131) subprotocol Q': [Annals of Oncology 30 (2019) 1821-1830].Ann Oncol. 2021 Aug;32(8):1068. doi: 10.1016/j.annonc.2021.05.797. Epub 2021 Jun 5. Ann Oncol. 2021. PMID: 34099371 Free PMC article. No abstract available.
Abstract
Background: The National Cancer Institute-Molecular Analysis for Therapy Choice (NCI-MATCH) is a national precision medicine study incorporating centralized genomic testing to direct refractory cancer patients to molecularly targeted treatment subprotocols. This treatment subprotocol was designed to screen for potential signals of efficacy of ado-trastuzumab emtansine (T-DM1) in HER2-amplified histologies other than breast and gastroesophageal tumors.
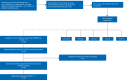
Methods: Eligible patients had HER2 amplification at a copy number (CN) >7 based on targeted next-generation sequencing (NGS) with a custom Oncomine AmpliSeq™ (ThermoFisher Scientific) panel. Patients with prior trastuzumab, pertuzumab or T-DM1 treatment were excluded. Patients received T-DM1 at 3.6 mg/kg i.v. every 3 weeks until toxicity or disease progression. Tumor assessments occurred every three cycles. The primary end point was centrally assessed objective response rate (ORR). Exploratory end points included correlating response with HER2 CN by NGS. The impact of co-occurring genomic alterations and PTEN loss by immunohistochemistry were also assessed.
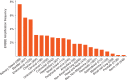
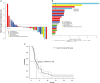
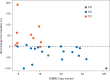
Results: Thirty-eight patients were enrolled and 36 included in efficacy analysis. Median prior therapies in the metastatic setting was 3 (range 0-9; unknown in one patient). Median HER2 CN was 17 (range 7-139). Partial responses were observed in two (5.6%) patients: one mucoepidermoid carcinoma of parotid gland and one parotid gland squamous cell cancer. Seventeen patients (47%) had stable disease including 8/10 (80%) with ovarian and uterine carcinomas, with median duration of 4.6 months. The 6-month progression-free survival rate was 23.6% [90% confidence interval 14.2% to 39.2%]. Common toxicities included fatigue, anemia, fever and thrombocytopenia with no new safety signals. There was a trend for tumor shrinkage with higher levels of gene CN as determined by the NGS assay.
Conclusion: T-DM1 was well tolerated. While this subprotocol did not meet the primary end point for ORR in this heavily pre-treated diverse patient population, clinical activity was seen in salivary gland tumors warranting further study in this tumor type in dedicated trials.
Keywords: HER2-amplified; NCI-MATCH; NGS; T-DM1.
© The Author(s) 2019. Published by Oxford University Press on behalf of the European Society for Medical Oncology. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures

Similar articles
-
Ado-trastuzumab for the treatment of metastatic HER2-positive breast cancer in patients previously treated with Pertuzumab.BMC Cancer. 2021 Oct 27;21(1):1150. doi: 10.1186/s12885-021-08894-2. BMC Cancer. 2021. PMID: 34706686 Free PMC article.
-
Trastuzumab and Pertuzumab in Patients with Non-Breast/Gastroesophageal HER2-Amplified Tumors: Results from the NCI-MATCH ECOG-ACRIN Trial (EAY131) Subprotocol J.Clin Cancer Res. 2024 Apr 1;30(7):1273-1280. doi: 10.1158/1078-0432.CCR-23-0633. Clin Cancer Res. 2024. PMID: 38433347 Free PMC article. Clinical Trial.
-
Clinical benefit of treatment after trastuzumab emtansine for HER2-positive metastatic breast cancer: a real-world multi-centre cohort study in Japan (WJOG12519B).Breast Cancer. 2021 May;28(3):581-591. doi: 10.1007/s12282-020-01192-y. Epub 2021 Jan 2. Breast Cancer. 2021. PMID: 33389616 Free PMC article.
-
HER2-Overexpressing/Amplified Breast Cancer as a Testing Ground for Antibody-Drug Conjugate Drug Development in Solid Tumors.Clin Cancer Res. 2020 Feb 15;26(4):775-786. doi: 10.1158/1078-0432.CCR-18-1976. Epub 2019 Oct 3. Clin Cancer Res. 2020. PMID: 31582515 Review.
-
Mechanisms of resistance to trastuzumab emtansine (T-DM1) in HER2-positive breast cancer.Br J Cancer. 2020 Mar;122(5):603-612. doi: 10.1038/s41416-019-0635-y. Epub 2019 Dec 16. Br J Cancer. 2020. PMID: 31839676 Free PMC article. Review.
Cited by
-
Survival benefit of HER2-targeted or androgen deprivation therapy in salivary duct carcinoma.Ther Adv Med Oncol. 2022 Sep 6;14:17588359221119538. doi: 10.1177/17588359221119538. eCollection 2022. Ther Adv Med Oncol. 2022. PMID: 36090801 Free PMC article.
-
Response to Anti-HER2-Based Treatment in a Patient with Bladder Adenocarcinoma Harboring HER2 Amplification and S310F Mutation Discovered by Next-Generation Sequencing: A Case Report.Onco Targets Ther. 2020 May 18;13:4249-4255. doi: 10.2147/OTT.S247515. eCollection 2020. Onco Targets Ther. 2020. PMID: 32547059 Free PMC article.
-
Clinical trial design in the era of precision medicine.Genome Med. 2022 Aug 31;14(1):101. doi: 10.1186/s13073-022-01102-1. Genome Med. 2022. PMID: 36045401 Free PMC article. Review.
-
Retrospective Study on the Efficacy and Safety of Pyrotinib-Based Therapy for HER2-Positive Nonbreast Advanced Solid Tumors.J Oncol. 2022 Mar 25;2022:4233782. doi: 10.1155/2022/4233782. eCollection 2022. J Oncol. 2022. PMID: 35368895 Free PMC article.
-
Functional Precision Medicine Provides Clinical Benefit in Advanced Aggressive Hematologic Cancers and Identifies Exceptional Responders.Cancer Discov. 2022 Feb;12(2):372-387. doi: 10.1158/2159-8290.CD-21-0538. Epub 2021 Oct 11. Cancer Discov. 2022. PMID: 34635570 Free PMC article.
References
-
- Slamon DJ, Clark GM, Wong SG. et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987; 235(4785): 177–182. - PubMed
-
- Slamon DJ, Godolphin W, Jones LA. et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 1989; 244(4905): 707–712. - PubMed
-
- Wolff AC, Hammond ME, Hicks DG. et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. JCO 2013; 31(31): 3997–4013. - PubMed
-
- Bang YJ, Van Cutsem E, Feyereislova A. et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010; 376(9742): 687–697. - PubMed
-
- Slamon DJ, Leyland-Jones B, Shak S. et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001; 344(11): 783–792. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

