Structural insights into TRPM8 inhibition and desensitization
- PMID: 31488702
- PMCID: PMC7262954
- DOI: 10.1126/science.aax6672
Structural insights into TRPM8 inhibition and desensitization
Abstract
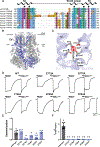
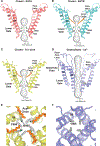
The transient receptor potential melastatin 8 (TRPM8) ion channel is the primary detector of environmental cold and an important target for treating pathological cold hypersensitivity. Here, we present cryo-electron microscopy structures of TRPM8 in ligand-free, antagonist-bound, or calcium-bound forms, revealing how robust conformational changes give rise to two nonconducting states, closed and desensitized. We describe a malleable ligand-binding pocket that accommodates drugs of diverse chemical structures, and we delineate the ion permeation pathway, including the contribution of lipids to pore architecture. Furthermore, we show that direct calcium binding mediates stimulus-evoked desensitization, clarifying this important mechanism of sensory adaptation. We observe large rearrangements within the S4-S5 linker that reposition the S1-S4 and pore domains relative to the TRP helix, leading us to propose a distinct model for modulation of TRPM8 and possibly other TRP channels.
Copyright © 2019 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures



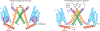
Similar articles
-
The region adjacent to the C-end of the inner gate in transient receptor potential melastatin 8 (TRPM8) channels plays a central role in allosteric channel activation.J Biol Chem. 2014 Oct 10;289(41):28579-94. doi: 10.1074/jbc.M114.577478. Epub 2014 Aug 25. J Biol Chem. 2014. PMID: 25157108 Free PMC article.
-
Phosphoinositide-interacting regulator of TRP (PIRT) has opposing effects on human and mouse TRPM8 ion channels.J Biol Chem. 2018 Jun 15;293(24):9423-9434. doi: 10.1074/jbc.RA118.003563. Epub 2018 May 3. J Biol Chem. 2018. PMID: 29724821 Free PMC article.
-
Structural basis of cooling agent and lipid sensing by the cold-activated TRPM8 channel.Science. 2019 Mar 1;363(6430):eaav9334. doi: 10.1126/science.aav9334. Epub 2019 Feb 7. Science. 2019. PMID: 30733385 Free PMC article.
-
Current View of Ligand and Lipid Recognition by the Menthol Receptor TRPM8.Trends Biochem Sci. 2020 Sep;45(9):806-819. doi: 10.1016/j.tibs.2020.05.008. Epub 2020 Jun 9. Trends Biochem Sci. 2020. PMID: 32532587 Free PMC article. Review.
-
Intimacies and physiological role of the polymodal cold-sensitive ion channel TRPM8.Curr Top Membr. 2014;74:293-324. doi: 10.1016/B978-0-12-800181-3.00011-7. Curr Top Membr. 2014. PMID: 25366241 Review.
Cited by
-
Structural basis for human TRPC5 channel inhibition by two distinct inhibitors.Elife. 2021 Mar 8;10:e63429. doi: 10.7554/eLife.63429. Elife. 2021. PMID: 33683200 Free PMC article.
-
Mechanisms of sensory adaptation and inhibition of the cold and menthol receptor TRPM8.Sci Adv. 2024 Aug 2;10(31):eadp2211. doi: 10.1126/sciadv.adp2211. Epub 2024 Aug 2. Sci Adv. 2024. PMID: 39093967 Free PMC article.
-
Mutations of TRPM8 channels: Unraveling the molecular basis of activation by cold and ligands.Med Res Rev. 2022 Nov;42(6):2168-2203. doi: 10.1002/med.21920. Epub 2022 Aug 17. Med Res Rev. 2022. PMID: 35976012 Free PMC article. Review.
-
Ligand-Binding Sites in Vanilloid-Subtype TRP Channels.Front Pharmacol. 2022 May 16;13:900623. doi: 10.3389/fphar.2022.900623. eCollection 2022. Front Pharmacol. 2022. PMID: 35652046 Free PMC article. Review.
-
Interaction of Calmodulin with TRPM: An Initiator of Channel Modulation.Int J Mol Sci. 2023 Oct 13;24(20):15162. doi: 10.3390/ijms242015162. Int J Mol Sci. 2023. PMID: 37894842 Free PMC article. Review.
References
-
- McKemy DD, Neuhausser WM, Julius D, Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 416, 52–58 (2002). - PubMed
-
- Peier AM et al., A TRP Channel that Senses Cold Stimuli and Menthol. Cell 108, 705–715 (2002). - PubMed
-
- Bautista DM et al., The menthol receptor TRPM8 is the principal detector of environmental cold. Nature 448, 204–208 (2007). - PubMed
-
- Dhaka A et al., TRPM8 Is Required for Cold Sensation in Mice. Neuron 54, 371–378 (2007). - PubMed
-
- Colburn RW et al., Attenuated Cold Sensitivity in TRPM8 Null Mice. Neuron 54, 379–386 (2007). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

