Phase II Study of Lenvatinib in Patients With Progressive, Recurrent or Metastatic Adenoid Cystic Carcinoma
- PMID: 30939095
- PMCID: PMC6599407
- DOI: 10.1200/JCO.18.01859
Phase II Study of Lenvatinib in Patients With Progressive, Recurrent or Metastatic Adenoid Cystic Carcinoma
Abstract
Purpose: Recurrent or metastatic adenoid cystic carcinoma (R/M ACC) is a malignant neoplasm of predominantly salivary gland origin for which effective therapies are lacking. We conducted a phase II trial evaluating the multitargeted tyrosine kinase inhibitor lenvatinib in patients with R/M ACC.
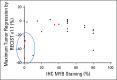
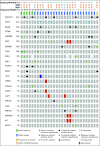
Patients and methods: This study was conducted with a two-stage minimax design. Patients with histologically confirmed R/M ACC of any primary site with radiographic and/or symptomatic progression were eligible. Any prior therapy was allowed except previous lenvatinib. Patients received lenvatinib 24 mg orally per day. The primary end point was overall response rate. Secondary end points were progression-free survival and safety. An exploratory analysis of how MYB expression and genomic alterations relate to outcomes was conducted.
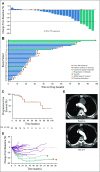
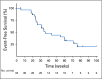
Results: Thirty-three patients were enrolled; 32 were evaluable for the primary end point. Five patients (15.6%) had a confirmed partial response, 24 patients (75%) had stable disease, two patients (6.3%) discontinued treatment as a result of toxicity before the first scan, and one patient (3.1%) had progression of disease as best response. Median progression-free survival time was 17.5 months (95% CI, 7.2 months to not reached), although only eight progression events were observed. Patients otherwise were removed for toxicity (n = 5), as a result of withdrawal of consent (n = 9), or at the treating physician's discretion (n = 6). Twenty-three patients required at least one dose modification, and 18 of 32 patients discontinued lenvatinib for drug-related issues. The most common grade 3 or 4 adverse events were hypertension (n = 9; 28.1%) and oral pain (n = 3; 9.4%). Three grade 4 adverse events were observed (myocardial infarction, n = 1; posterior reversible encephalopathy syndrome, n = 1; and intracranial hemorrhage, n = 1).
Conclusion: This trial met the prespecified overall response rate primary end point, demonstrating antitumor activity with lenvatinib in R/M ACC patients. Toxicity was comparable to previous studies, requiring monitoring and management.
Figures
Similar articles
-
Patients with adenoid cystic carcinomas of the salivary glands treated with lenvatinib: Activity and quality of life.Cancer. 2020 Jan 1;126(9):1888-1894. doi: 10.1002/cncr.32754. Epub 2020 Feb 7. Cancer. 2020. PMID: 32031693 Clinical Trial.
-
A phase II study of sorafenib in recurrent and/or metastatic salivary gland carcinomas: Translational analyses and clinical impact.Eur J Cancer. 2016 Dec;69:158-165. doi: 10.1016/j.ejca.2016.09.022. Epub 2016 Nov 5. Eur J Cancer. 2016. PMID: 27821319 Clinical Trial.
-
A phase II study of axitinib (AG-013736) in patients with incurable adenoid cystic carcinoma.Ann Oncol. 2016 Oct;27(10):1902-8. doi: 10.1093/annonc/mdw287. Epub 2016 Aug 26. Ann Oncol. 2016. PMID: 27566443 Free PMC article. Clinical Trial.
-
Lenvatinib: Role in thyroid cancer and other solid tumors.Cancer Treat Rev. 2016 Jan;42:47-55. doi: 10.1016/j.ctrv.2015.11.003. Epub 2015 Dec 2. Cancer Treat Rev. 2016. PMID: 26678514 Review.
-
Lenvatinib in Advanced, Radioactive Iodine-Refractory, Differentiated Thyroid Carcinoma.Clin Cancer Res. 2015 Dec 15;21(24):5420-6. doi: 10.1158/1078-0432.CCR-15-0923. Epub 2015 Oct 20. Clin Cancer Res. 2015. PMID: 26487760 Review.
Cited by
-
Approaches to the Management of Metastatic Adenoid Cystic Carcinoma.Cancers (Basel). 2022 Nov 20;14(22):5698. doi: 10.3390/cancers14225698. Cancers (Basel). 2022. PMID: 36428790 Free PMC article. Review.
-
Multicentre, retrospective study of the efficacy and safety of nivolumab for recurrent and metastatic salivary gland carcinoma.Sci Rep. 2020 Oct 12;10(1):16988. doi: 10.1038/s41598-020-73965-6. Sci Rep. 2020. PMID: 33046752 Free PMC article.
-
Adenoid cystic carcinoma: insights from molecular characterization and therapeutic advances.MedComm (2020). 2024 Sep 11;5(9):e734. doi: 10.1002/mco2.734. eCollection 2024 Sep. MedComm (2020). 2024. PMID: 39263605 Free PMC article. Review.
-
How predominant cell and stroma types harmonize to predict head and neck adenoid cystic carcinoma outcomes?Med J Armed Forces India. 2024 Jul-Aug;80(4):404-411. doi: 10.1016/j.mjafi.2024.05.012. Epub 2024 Jun 21. Med J Armed Forces India. 2024. PMID: 39071760 Review.
-
PSMA-Directed Imaging and Therapy of Salivary Gland Tumors: A Single-Center Retrospective Study.J Nucl Med. 2023 Mar;64(3):372-378. doi: 10.2967/jnumed.122.264342. Epub 2022 Sep 22. J Nucl Med. 2023. PMID: 36137757 Free PMC article.
References
-
- Spiro RH. Distant metastasis in adenoid cystic carcinoma of salivary origin. Am J Surg. 1997;174:495–498. - PubMed
-
- Chau NG, Hotte SJ, Chen EX, et al. A phase II study of sunitinib in recurrent and/or metastatic adenoid cystic carcinoma (ACC) of the salivary glands: Current progress and challenges in evaluating molecularly targeted agents in ACC. Ann Oncol. 2012;23:1562–1570. - PubMed
-
- Thomson DJ, Silva P, Denton K, et al. Phase II trial of sorafenib in advanced salivary adenoid cystic carcinoma of the head and neck. Head Neck. 2015;37:182–187. - PubMed
-
- Locati LD, Perrone F, Cortelazzi B, et al. A phase II study of sorafenib in recurrent and/or metastatic salivary gland carcinomas: Translational analyses and clinical impact. Eur J Cancer. 2016;69:158–165. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

