Human cytomegalovirus-infected cells release extracellular vesicles that carry viral surface proteins
- PMID: 30165311
- PMCID: PMC6258833
- DOI: 10.1016/j.virol.2018.08.008
Human cytomegalovirus-infected cells release extracellular vesicles that carry viral surface proteins
Abstract
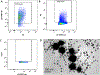
Extracellular vesicles (EVs) released by virus-infected cells typically incorporate host and viral components inside the vesicles (cargo molecules). Here, we investigated if human cytomegalovirus (HCMV) proteins are incorporated in EV outer membrane released by HCMV-infected cells. We separated EVs from HCMV using an iodixanol step-gradient and found that the separated vesicles carried EV markers such as the tetraspanin CD63 and Rab27A. Flow analysis of individual EVs demonstrated that on average, 15 ± 3.7% of EVs were positive for gB, 5.3 ± 2.3% were positive for gH and 3.74 ± 1.5% were positive for both gB and gH. In light of previous findings demonstrating HIV envelope proteins in EV membranes, the presence of viral protein at the surface of EVs released by HCMV-infected cells indicated that viral membrane proteins incorporated in EVs released by virus-infected cells may be a general phenomenon.
Keywords: Extracellular vesicles; HCMV; gB; gH.
Published by Elsevier Inc.
Conflict of interest statement
Conflict of interests
The authors declare that they have no competing financial interests.
Figures



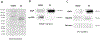
Similar articles
-
Human Cytomegalovirus Utilizes Extracellular Vesicles To Enhance Virus Spread.J Virol. 2020 Jul 30;94(16):e00609-20. doi: 10.1128/JVI.00609-20. Print 2020 Jul 30. J Virol. 2020. PMID: 32522858 Free PMC article.
-
Extracellular Vesicles Released by Herpes Simplex Virus 1-Infected Cells Block Virus Replication in Recipient Cells in a STING-Dependent Manner.J Virol. 2018 Aug 29;92(18):e01102-18. doi: 10.1128/JVI.01102-18. Print 2018 Sep 15. J Virol. 2018. PMID: 29976662 Free PMC article.
-
Diverse Populations of Extracellular Vesicles with Opposite Functions during Herpes Simplex Virus 1 Infection.J Virol. 2021 Feb 24;95(6):e02357-20. doi: 10.1128/JVI.02357-20. Print 2021 Feb 24. J Virol. 2021. PMID: 33361424 Free PMC article.
-
Delivery of microRNAs by Extracellular Vesicles in Viral Infections: Could the News be Packaged?Cells. 2019 Jun 18;8(6):611. doi: 10.3390/cells8060611. Cells. 2019. PMID: 31216738 Free PMC article. Review.
-
Human cytomegalovirus glycoproteins.Intervirology. 1996;39(5-6):401-12. doi: 10.1159/000150510. Intervirology. 1996. PMID: 9130049 Review.
Cited by
-
Intermittent bulk release of human cytomegalovirus.PLoS Pathog. 2022 Aug 4;18(8):e1010575. doi: 10.1371/journal.ppat.1010575. eCollection 2022 Aug. PLoS Pathog. 2022. PMID: 35925870 Free PMC article.
-
Intercellular Transport of Viral Proteins.Results Probl Cell Differ. 2024;73:435-474. doi: 10.1007/978-3-031-62036-2_18. Results Probl Cell Differ. 2024. PMID: 39242389 Review.
-
Prokaryotic microvesicles Ortholog of eukaryotic extracellular vesicles in biomedical fields.Cell Commun Signal. 2024 Jan 30;22(1):80. doi: 10.1186/s12964-023-01414-8. Cell Commun Signal. 2024. PMID: 38291458 Free PMC article. Review.
-
Exploring extracellular vesicles as mediators of clinical disease and vehicles for viral therapeutics: Insights from the COVID-19 pandemic.Extracell Vesicles Circ Nucl Acids. 2022;3(3):172-188. doi: 10.20517/evcna.2022.19. Epub 2022 Jul 19. Extracell Vesicles Circ Nucl Acids. 2022. PMID: 35929616 Free PMC article.
-
The host exosome pathway underpins biogenesis of the human cytomegalovirus virion.Elife. 2020 Sep 10;9:e58288. doi: 10.7554/eLife.58288. Elife. 2020. PMID: 32910773 Free PMC article.
References
-
- Arvin A, Campadelli-Fiume G, Mocarski E, Moore P, Roizman B, Whitley R, Yamanishi K, 2007. Human Herpesviruses. Cambridge University Press. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

