A genetically encoded fluorescent acetylcholine indicator for in vitro and in vivo studies
- PMID: 29985477
- PMCID: PMC6093211
- DOI: 10.1038/nbt.4184
A genetically encoded fluorescent acetylcholine indicator for in vitro and in vivo studies
Abstract
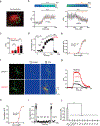
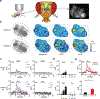
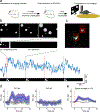
The neurotransmitter acetylcholine (ACh) regulates a diverse array of physiological processes throughout the body. Despite its importance, cholinergic transmission in the majority of tissues and organs remains poorly understood owing primarily to the limitations of available ACh-monitoring techniques. We developed a family of ACh sensors (GACh) based on G-protein-coupled receptors that has the sensitivity, specificity, signal-to-noise ratio, kinetics and photostability suitable for monitoring ACh signals in vitro and in vivo. GACh sensors were validated with transfection, viral and/or transgenic expression in a dozen types of neuronal and non-neuronal cells prepared from multiple animal species. In all preparations, GACh sensors selectively responded to exogenous and/or endogenous ACh with robust fluorescence signals that were captured by epifluorescence, confocal, and/or two-photon microscopy. Moreover, analysis of endogenous ACh release revealed firing-pattern-dependent release and restricted volume transmission, resolving two long-standing questions about central cholinergic transmission. Thus, GACh sensors provide a user-friendly, broadly applicable tool for monitoring cholinergic transmission underlying diverse biological processes.
Figures



Similar articles
-
An optimized acetylcholine sensor for monitoring in vivo cholinergic activity.Nat Methods. 2020 Nov;17(11):1139-1146. doi: 10.1038/s41592-020-0953-2. Epub 2020 Sep 28. Nat Methods. 2020. PMID: 32989318 Free PMC article.
-
Comparison of fluorescence biosensors and whole-cell patch clamp recording in detecting ACh, NE, and 5-HT.Front Cell Neurosci. 2023 Jun 2;17:1166480. doi: 10.3389/fncel.2023.1166480. eCollection 2023. Front Cell Neurosci. 2023. PMID: 37333890 Free PMC article.
-
How can I measure brain acetylcholine levels in vivo? Advantages and caveats of commonly used approaches.J Neurochem. 2023 Oct;167(1):3-15. doi: 10.1111/jnc.15943. Epub 2023 Aug 24. J Neurochem. 2023. PMID: 37621094 Free PMC article. Review.
-
Evoked acetylcholine release by immortalized brain endothelial cells genetically modified to express choline acetyltransferase and/or the vesicular acetylcholine transporter.J Neurochem. 1999 Oct;73(4):1483-91. doi: 10.1046/j.1471-4159.1999.0731483.x. J Neurochem. 1999. PMID: 10501193
-
Cotransmission of acetylcholine and GABA.Neuropharmacology. 2016 Jan;100:40-6. doi: 10.1016/j.neuropharm.2015.07.031. Epub 2015 Jul 26. Neuropharmacology. 2016. PMID: 26220313 Free PMC article. Review.
Cited by
-
Neuromodulator and neuropeptide sensors and probes for precise circuit interrogation in vivo.Science. 2024 Sep 27;385(6716):eadn6671. doi: 10.1126/science.adn6671. Epub 2024 Sep 27. Science. 2024. PMID: 39325905 Free PMC article. Review.
-
Acetylcholine is released in the basolateral amygdala in response to predictors of reward and enhances the learning of cue-reward contingency.Elife. 2020 Sep 18;9:e57335. doi: 10.7554/eLife.57335. Elife. 2020. PMID: 32945260 Free PMC article.
-
Structural and pharmacological insights into cordycepin for neoplasms and metabolic disorders.Front Pharmacol. 2024 Jun 17;15:1367820. doi: 10.3389/fphar.2024.1367820. eCollection 2024. Front Pharmacol. 2024. PMID: 38953102 Free PMC article. Review.
-
Exploiting the molecular diversity of the synapse to investigate neuronal communication: A guide through the current toolkit.Eur J Neurosci. 2022 Dec;56(12):6141-6161. doi: 10.1111/ejn.15848. Epub 2022 Nov 4. Eur J Neurosci. 2022. PMID: 36239030 Free PMC article. Review.
-
The endocrinology of the brain.Endocr Connect. 2018 Dec 1;7(12):R275-R285. doi: 10.1530/EC-18-0367. Endocr Connect. 2018. PMID: 30352398 Free PMC article. Review.
References
-
- Dani JA & Bertrand D Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annual review of pharmacology and toxicology 47, 699–729 (2007). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

