Tumor Mutational Burden and Efficacy of Nivolumab Monotherapy and in Combination with Ipilimumab in Small-Cell Lung Cancer
- PMID: 29731394
- PMCID: PMC6750707
- DOI: 10.1016/j.ccell.2018.04.001
Tumor Mutational Burden and Efficacy of Nivolumab Monotherapy and in Combination with Ipilimumab in Small-Cell Lung Cancer
Erratum in
-
Tumor Mutational Burden and Efficacy of Nivolumab Monotherapy and in Combination with Ipilimumab in Small-Cell Lung Cancer.Cancer Cell. 2019 Feb 11;35(2):329. doi: 10.1016/j.ccell.2019.01.011. Cancer Cell. 2019. PMID: 30753829 No abstract available.
Abstract
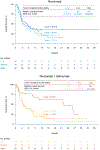
Durable responses and encouraging survival have been demonstrated with immune checkpoint inhibitors in small-cell lung cancer (SCLC), but predictive markers are unknown. We used whole exome sequencing to evaluate the impact of tumor mutational burden on efficacy of nivolumab monotherapy or combined with ipilimumab in patients with SCLC from the nonrandomized or randomized cohorts of CheckMate 032. Patients received nivolumab (3 mg/kg every 2 weeks) or nivolumab plus ipilimumab (1 mg/kg plus 3 mg/kg every 3 weeks for four cycles, followed by nivolumab 3 mg/kg every 2 weeks). Efficacy of nivolumab ± ipilimumab was enhanced in patients with high tumor mutational burden. Nivolumab plus ipilimumab appeared to provide a greater clinical benefit than nivolumab monotherapy in the high tumor mutational burden tertile.
Keywords: CTLA-4; PD-1; biomarkers; clinical trial; immunotherapy; ipilimumab; nivolumab; small cell lung cancer; tumor mutation burden.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
Matthew D. Hellmann reports paid consultancy from AstraZeneca/MedImmune, Bristol-Myers Squibb, Genentech, Janssen, Merck, Mirati Therapeutics, Novartis, Shattuck Labs, research funding from Bristol-Myers Squibb, and patent filed by Memorial Sloan Kettering related to the use of tumor mutation burden to predict response to immunotherapy (PCT/US2015/062208); Margaret K. Callahan reports grants from and employment of a family member by Bristol-Myers Squibb; personal fees for advisory board participation from AstraZeneca and Incyte; Mark M. Awad reports consulting or advisory role for AbbVie, AstraZeneca, Boehringer Ingelheim, Genentech, Merck, and Pfizer; Paolo A. Ascierto reports grants for research funding and personal fees for advisory/consultant role from Array, Bristol-Myers Squibb, and Roche-Genentech, and personal fees for advisory/consultant role from Amgen, Genmab, Incyte, Medimmune, Merck Serono, NewLink Genetics, Novartis, and Pierre Fabre; Akin Atmaca reports travel grants and honoraria for advisory board participation from Bristol-Myers Squibb, Merck Sharp & Dohme, and Roche; Naiyer A. Rizvi reports a leadership role with ARMO Biosciences, stock or other ownership from ARMO Biosciences and Gritstone Oncology, consulting or advisory role for AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Genentech, MedImmune, Merck Sharp & Dohme, Novartis, Pfizer, and Roche; Giovanni Selvaggi reports employment by Bristol-Myers Squibb; Joseph D. Szustakowski reports employment and stock ownership from Bristol-Myers Squibb and previous employment and stock ownership from Novartis; Ariella Sasson reports employment by Bristol-Myers Squibb; Ryan Golhar reports employment by Bristol-Myers Squibb; Patrik Vitazka reports employment by Bristol-Myers Squibb; Han Chang reports employment by Bristol-Myers Squibb; William J. Geese reports employment and stock ownership from Bristol-Myers Squibb; Scott J. Antonia reports stock or other ownership from Cellular Biomedicine Group, and honoraria, consulting or advisory role, travel, accommodations, and expenses from AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, and Merck; all other authors report nothing to disclose.
Figures

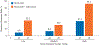


Comment in
-
Genomic Features of Response to Combination Immunotherapy in Lung Cancer.Cancer Cell. 2018 May 14;33(5):791-793. doi: 10.1016/j.ccell.2018.04.005. Cancer Cell. 2018. PMID: 29763618
-
Tumor mutational burden (TMB) as a biomarker of response to immunotherapy in small cell lung cancer.J Thorac Dis. 2018 Aug;10(8):4689-4693. doi: 10.21037/jtd.2018.07.120. J Thorac Dis. 2018. PMID: 30233840 Free PMC article. No abstract available.
Similar articles
-
Nivolumab and Ipilimumab as Maintenance Therapy in Extensive-Disease Small-Cell Lung Cancer: CheckMate 451.J Clin Oncol. 2021 Apr 20;39(12):1349-1359. doi: 10.1200/JCO.20.02212. Epub 2021 Mar 8. J Clin Oncol. 2021. PMID: 33683919 Free PMC article. Clinical Trial.
-
First-Line Nivolumab Plus Ipilimumab in Advanced Non-Small-Cell Lung Cancer (CheckMate 568): Outcomes by Programmed Death Ligand 1 and Tumor Mutational Burden as Biomarkers.J Clin Oncol. 2019 Apr 20;37(12):992-1000. doi: 10.1200/JCO.18.01042. Epub 2019 Feb 20. J Clin Oncol. 2019. PMID: 30785829 Free PMC article. Clinical Trial.
-
Nivolumab plus Ipilimumab in Lung Cancer with a High Tumor Mutational Burden.N Engl J Med. 2018 May 31;378(22):2093-2104. doi: 10.1056/NEJMoa1801946. Epub 2018 Apr 16. N Engl J Med. 2018. PMID: 29658845 Free PMC article. Clinical Trial.
-
Role of Immune Checkpoint Inhibitors in Small Cell Lung Cancer.Am J Ther. 2018 May/Jun;25(3):e349-e356. doi: 10.1097/MJT.0000000000000686. Am J Ther. 2018. PMID: 29722737 Review.
-
PD-1 checkpoint blockade alone or combined PD-1 and CTLA-4 blockade as immunotherapy for lung cancer?Expert Opin Biol Ther. 2017 Mar;17(3):305-312. doi: 10.1080/14712598.2017.1280454. Epub 2017 Jan 18. Expert Opin Biol Ther. 2017. PMID: 28064556 Review.
Cited by
-
Treatment-Related Serious Adverse Events of Immune Checkpoint Inhibitors in Clinical Trials: A Systematic Review.Front Oncol. 2021 May 11;11:621639. doi: 10.3389/fonc.2021.621639. eCollection 2021. Front Oncol. 2021. PMID: 34046338 Free PMC article.
-
Immune-Related Adverse Events Predict the Efficacy of Immune Checkpoint Inhibitors in Lung Cancer Patients: A Meta-Analysis.Front Oncol. 2021 Mar 1;11:631949. doi: 10.3389/fonc.2021.631949. eCollection 2021. Front Oncol. 2021. PMID: 33732650 Free PMC article.
-
Meta-analysis of immune-related adverse events of immune checkpoint inhibitor therapy in cancer patients.Thorac Cancer. 2020 Sep;11(9):2406-2430. doi: 10.1111/1759-7714.13541. Epub 2020 Jul 8. Thorac Cancer. 2020. PMID: 32643323 Free PMC article.
-
Tumor mutation burden in Chinese cancer patients and the underlying driving pathways of high tumor mutation burden across different cancer types.Ann Transl Med. 2020 Jul;8(14):860. doi: 10.21037/atm-20-3807. Ann Transl Med. 2020. PMID: 32793704 Free PMC article.
-
Inhibiting the MNK1/2-eIF4E axis impairs melanoma phenotype switching and potentiates antitumor immune responses.J Clin Invest. 2021 Apr 15;131(8):e140752. doi: 10.1172/JCI140752. J Clin Invest. 2021. PMID: 33690225 Free PMC article.
References
-
- Antonia SJ, López-Martin JA, Bendell J, Ott PA, Taylor M, Eder JP, Jäger D, Pietanza MC, Le DT, de Braud F, et al. (2016). Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. The Lancet. Oncology 17, 883–895. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

