Safety and Efficacy of Pembrolizumab Monotherapy in Patients With Previously Treated Advanced Gastric and Gastroesophageal Junction Cancer: Phase 2 Clinical KEYNOTE-059 Trial
- PMID: 29543932
- PMCID: PMC5885175
- DOI: 10.1001/jamaoncol.2018.0013
Safety and Efficacy of Pembrolizumab Monotherapy in Patients With Previously Treated Advanced Gastric and Gastroesophageal Junction Cancer: Phase 2 Clinical KEYNOTE-059 Trial
Erratum in
-
Incomplete Conflict of Interest Disclosures.JAMA Oncol. 2019 Apr 1;5(4):579. doi: 10.1001/jamaoncol.2019.0286. JAMA Oncol. 2019. PMID: 30844032 Free PMC article. No abstract available.
Abstract
Importance: Therapeutic options are needed for patients with advanced gastric cancer whose disease has progressed after 2 or more lines of therapy.
Objective: To evaluate the safety and efficacy of pembrolizumab in a cohort of patients with previously treated gastric or gastroesophageal junction cancer.
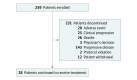
Design, setting, and participants: In the phase 2, global, open-label, single-arm, multicohort KEYNOTE-059 study, 259 patients in 16 countries were enrolled in a cohort between March 2, 2015, and May 26, 2016. Median (range) follow-up was 5.8 (0.5-21.6) months.
Intervention: Patients received pembrolizumab, 200 mg, intravenously every 3 weeks until disease progression, investigator or patient decision to withdraw, or unacceptable toxic effects.
Main outcomes and measures: Primary end points were objective response rate and safety. Objective response rate was assessed by central radiologic review per Response Evaluation Criteria in Solid Tumors, version 1.1, in all patients and those with programmed cell death 1 ligand 1 (PD-L1)-positive tumors. Expression of PD-L1 was assessed by immunohistochemistry. Secondary end points included response duration.
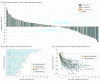
Results: Of 259 patients enrolled, most were male (198 [76.4%]) and white (200 [77.2%]); median (range) age was 62 (24-89) years. Objective response rate was 11.6% (95% CI, 8.0%-16.1%; 30 of 259 patients), with complete response in 2.3% (95% CI, 0.9%-5.0%; 6 of 259 patients). Median (range) response duration was 8.4 (1.6+ to 17.3+) months (+ indicates that patients had no progressive disease at their last assessment). Objective response rate and median (range) response duration were 15.5% (95% CI, 10.1%-22.4%; 23 of 148 patients) and 16.3 (1.6+ to 17.3+) months and 6.4% (95% CI, 2.6%-12.8%; 7 of 109 patients) and 6.9 (2.4 to 7.0+) months in patients with PD-L1-positive and PD-L1-negative tumors, respectively. Forty-six patients (17.8%) experienced 1 or more grade 3 to 5 treatment-related adverse events. Two patients (0.8%) discontinued because of treatment-related adverse events, and 2 deaths were considered related to treatment.
Conclusions and relevance: Pembrolizumab monotherapy demonstrated promising activity and manageable safety in patients with advanced gastric or gastroesophageal junction cancer who had previously received at least 2 lines of treatment. Durable responses were observed in patients with PD-L1-positive and PD-L1-negative tumors. Further study of pembrolizumab for this group of patients is warranted.
Trial registration: clinicaltrials.gov Identifier: NCT02335411.
Conflict of interest statement
Figures
Comment in
-
Pembrolizumab in advanced gastric cancer: pioneering or prosaic?Ann Transl Med. 2019 Mar;7(Suppl 1):S3. doi: 10.21037/atm.2019.01.17. Ann Transl Med. 2019. PMID: 31032284 Free PMC article. No abstract available.
-
When survival curves cross: are we at a crossroads of immunotherapy in gastric cancer?Ann Transl Med. 2019 Mar;7(Suppl 1):S35. doi: 10.21037/atm.2019.02.13. Ann Transl Med. 2019. PMID: 31032314 Free PMC article. No abstract available.
Comment on
-
Omitted Disclosures of Potential Conflicts of Interest in Articles Published in JAMA Oncology.JAMA Oncol. 2019 Apr 1;5(4):578-579. doi: 10.1001/jamaoncol.2019.0235. JAMA Oncol. 2019. PMID: 30844040 No abstract available.
Similar articles
-
Efficacy and Safety of Pembrolizumab for Heavily Pretreated Patients With Advanced, Metastatic Adenocarcinoma or Squamous Cell Carcinoma of the Esophagus: The Phase 2 KEYNOTE-180 Study.JAMA Oncol. 2019 Apr 1;5(4):546-550. doi: 10.1001/jamaoncol.2018.5441. JAMA Oncol. 2019. PMID: 30570649 Free PMC article. Clinical Trial.
-
Safety and Antitumor Activity of the Anti-Programmed Death-1 Antibody Pembrolizumab in Patients With Advanced Esophageal Carcinoma.J Clin Oncol. 2018 Jan 1;36(1):61-67. doi: 10.1200/JCO.2017.74.9846. Epub 2017 Nov 8. J Clin Oncol. 2018. PMID: 29116900 Clinical Trial.
-
Ramucirumab plus pembrolizumab in patients with previously treated advanced non-small-cell lung cancer, gastro-oesophageal cancer, or urothelial carcinomas (JVDF): a multicohort, non-randomised, open-label, phase 1a/b trial.Lancet Oncol. 2019 Aug;20(8):1109-1123. doi: 10.1016/S1470-2045(19)30458-9. Epub 2019 Jul 10. Lancet Oncol. 2019. PMID: 31301962 Clinical Trial.
-
Pembrolizumab for treatment of advanced gastric and gastroesophageal junction adenocarcinoma.Future Oncol. 2018 Feb;14(5):417-430. doi: 10.2217/fon-2017-0436. Epub 2017 Nov 2. Future Oncol. 2018. PMID: 29094609 Free PMC article. Review.
-
Advances in immuno-oncology biomarkers for gastroesophageal cancer: Programmed death ligand 1, microsatellite instability, and beyond.World J Gastroenterol. 2018 Jul 7;24(25):2686-2697. doi: 10.3748/wjg.v24.i25.2686. World J Gastroenterol. 2018. PMID: 29991874 Free PMC article. Review.
Cited by
-
Margetuximab with retifanlimab as first-line therapy in HER2+/PD-L1+ unresectable or metastatic gastroesophageal adenocarcinoma: MAHOGANY cohort A.ESMO Open. 2022 Oct;7(5):100563. doi: 10.1016/j.esmoop.2022.100563. Epub 2022 Aug 24. ESMO Open. 2022. PMID: 36029651 Free PMC article.
-
Current status of esophageal cancer treatment.Chin J Cancer Res. 2020 Jun;32(3):271-286. doi: 10.21147/j.issn.1000-9604.2020.03.01. Chin J Cancer Res. 2020. PMID: 32694894 Free PMC article.
-
The Crosstalk between Microbiome and Immune Response in Gastric Cancer.Int J Mol Sci. 2020 Sep 9;21(18):6586. doi: 10.3390/ijms21186586. Int J Mol Sci. 2020. PMID: 32916853 Free PMC article. Review.
-
A novel artificial intelligence network to assess the prognosis of gastrointestinal cancer to immunotherapy based on genetic mutation features.Front Immunol. 2024 Jun 27;15:1428529. doi: 10.3389/fimmu.2024.1428529. eCollection 2024. Front Immunol. 2024. PMID: 38994371 Free PMC article.
-
Identification of potential biomarkers and their clinical significance in gastric cancer using bioinformatics analysis methods.PeerJ. 2020 Jul 14;8:e9174. doi: 10.7717/peerj.9174. eCollection 2020. PeerJ. 2020. PMID: 33062405 Free PMC article.
References
-
- Ferlay J, Soerjomataram I, Dikshit R, et al. . Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):-. - PubMed
-
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87-108. - PubMed
-
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69-90. - PubMed
-
- Wagner AD, Unverzagt S, Grothe W, et al. . Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev. 2010;(3):CD004064. - PubMed
-
- Lordick F, Shitara K, Janjigian YY. New agents on the horizon in gastric cancer. Ann Oncol. 2017;28(8):1767-1775. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

