Relationship of Sequence and Phase Separation in Protein Low-Complexity Regions
- PMID: 29517898
- PMCID: PMC6476794
- DOI: 10.1021/acs.biochem.8b00008
Relationship of Sequence and Phase Separation in Protein Low-Complexity Regions
Abstract
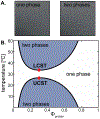
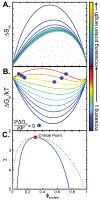
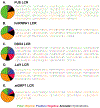
Liquid-liquid phase separation seems to play critical roles in the compartmentalization of cells through the formation of biomolecular condensates. Many proteins with low-complexity regions are found in these condensates, and they can undergo phase separation in vitro in response to changes in temperature, pH, and ion concentration. Low-complexity regions are thus likely important players in mediating compartmentalization in response to stress. However, how the phase behavior is encoded in their amino acid composition and patterning is only poorly understood. We discuss here that polymer physics provides a powerful framework for our understanding of the thermodynamics of mixing and demixing and for how the phase behavior is encoded in the primary sequence. We propose to classify low-complexity regions further into subcategories based on their sequence properties and phase behavior. Ongoing research promises to improve our ability to link the primary sequence of low-complexity regions to their phase behavior as well as the emerging miscibility and material properties of the resulting biomolecular condensates, providing mechanistic insight into this fundamental biological process across length scales.
Figures


Similar articles
-
Phase Separation: Linking Cellular Compartmentalization to Disease.Trends Cell Biol. 2016 Jul;26(7):547-558. doi: 10.1016/j.tcb.2016.03.004. Epub 2016 Apr 1. Trends Cell Biol. 2016. PMID: 27051975 Review.
-
The Control Centers of Biomolecular Phase Separation: How Membrane Surfaces, PTMs, and Active Processes Regulate Condensation.Mol Cell. 2019 Oct 17;76(2):295-305. doi: 10.1016/j.molcel.2019.09.016. Epub 2019 Oct 8. Mol Cell. 2019. PMID: 31604601 Free PMC article. Review.
-
Theories for Sequence-Dependent Phase Behaviors of Biomolecular Condensates.Biochemistry. 2018 May 1;57(17):2499-2508. doi: 10.1021/acs.biochem.8b00058. Epub 2018 Mar 13. Biochemistry. 2018. PMID: 29509422 Review.
-
How do intrinsically disordered protein regions encode a driving force for liquid-liquid phase separation?Curr Opin Struct Biol. 2021 Apr;67:41-50. doi: 10.1016/j.sbi.2020.09.004. Epub 2020 Oct 15. Curr Opin Struct Biol. 2021. PMID: 33069007 Free PMC article. Review.
-
Topological Considerations in Biomolecular Condensation.Biomolecules. 2023 Jan 11;13(1):151. doi: 10.3390/biom13010151. Biomolecules. 2023. PMID: 36671536 Free PMC article. Review.
Cited by
-
Electrostatic modulation of hnRNPA1 low-complexity domain liquid-liquid phase separation and aggregation.Protein Sci. 2021 Jul;30(7):1408-1417. doi: 10.1002/pro.4108. Epub 2021 May 22. Protein Sci. 2021. PMID: 33982369 Free PMC article.
-
Designing Electrostatic Interactions via Polyelectrolyte Monomer Sequence.ACS Cent Sci. 2019 Apr 24;5(4):709-718. doi: 10.1021/acscentsci.9b00087. Epub 2019 Apr 5. ACS Cent Sci. 2019. PMID: 31041391 Free PMC article.
-
Intrinsically Disordered Proteins (IDP): Purification Under Denaturing Conditions.Methods Mol Biol. 2022;2406:359-370. doi: 10.1007/978-1-0716-1859-2_21. Methods Mol Biol. 2022. PMID: 35089568
-
Driving forces behind phase separation of the carboxy-terminal domain of RNA polymerase II.Nat Commun. 2023 Sep 25;14(1):5979. doi: 10.1038/s41467-023-41633-8. Nat Commun. 2023. PMID: 37749095 Free PMC article.
-
Physical Chemistry of the Protein Backbone: Enabling the Mechanisms of Intrinsic Protein Disorder.J Phys Chem B. 2020 Jun 4;124(22):4379-4390. doi: 10.1021/acs.jpcb.0c02489. Epub 2020 May 14. J Phys Chem B. 2020. PMID: 32349480 Free PMC article. Review.
References
-
- Brangwynne CP, Eckmann CR, Courson DS, Rybarska A, Hoege C, Gharakhani J, Julicher F, and Hyman AA (2009) Germline P granules are liquid droplets that localize by controlled dissolution/condensation, Science 324, 1729–1732. - PubMed
-
- Hyman AA, and Brangwynne CP (2011) Beyond stereospecificity: liquids and mesoscale organization of cytoplasm, Dev Cell 21, 14–16. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

