Systems-wide Studies Uncover Commander, a Multiprotein Complex Essential to Human Development
- PMID: 28544880
- PMCID: PMC5541947
- DOI: 10.1016/j.cels.2017.04.006
Systems-wide Studies Uncover Commander, a Multiprotein Complex Essential to Human Development
Abstract
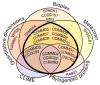
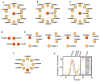
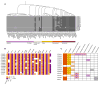
Recent mass spectrometry maps of the human interactome independently support the existence of a large multiprotein complex, dubbed "Commander." Broadly conserved across animals and ubiquitously expressed in nearly every human cell type examined thus far, Commander likely plays a fundamental cellular function, akin to other ubiquitous machines involved in expression, proteostasis, and trafficking. Experiments on individual subunits support roles in endosomal protein sorting, including the trafficking of Notch proteins, copper transporters, and lipoprotein receptors. Commander is critical for vertebrate embryogenesis, and defects in the complex and its interaction partners disrupt craniofacial, brain, and heart development. Here, we review the synergy between large-scale proteomic efforts and focused studies in the discovery of Commander, describe its composition, structure, and function, and discuss how it illustrates the power of systems biology. Based on 3D modeling and biochemical data, we draw strong parallels between Commander and the retromer cargo-recognition complex, laying a foundation for future research into Commander's role in human developmental disorders.
Keywords: Commander complex; developmental disorders; endosomal protein sorting; mammalian interactome; multiprotein complex; system-wide studies.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Commander Complex-A Multifaceted Operator in Intracellular Signaling and Cargo.Cells. 2021 Dec 7;10(12):3447. doi: 10.3390/cells10123447. Cells. 2021. PMID: 34943955 Free PMC article. Review.
-
Retromer and sorting nexins in endosomal sorting.Biochem Soc Trans. 2015 Feb;43(1):33-47. doi: 10.1042/BST20140290. Biochem Soc Trans. 2015. PMID: 25619244 Review.
-
Retriever is a multiprotein complex for retromer-independent endosomal cargo recycling.Nat Cell Biol. 2017 Oct;19(10):1214-1225. doi: 10.1038/ncb3610. Epub 2017 Sep 11. Nat Cell Biol. 2017. PMID: 28892079 Free PMC article.
-
Updated Insight into the Physiological and Pathological Roles of the Retromer Complex.Int J Mol Sci. 2017 Jul 25;18(8):1601. doi: 10.3390/ijms18081601. Int J Mol Sci. 2017. PMID: 28757549 Free PMC article. Review.
-
Structure and interactions of the endogenous human Commander complex.Nat Struct Mol Biol. 2024 Jun;31(6):925-938. doi: 10.1038/s41594-024-01246-1. Epub 2024 Mar 8. Nat Struct Mol Biol. 2024. PMID: 38459129 Free PMC article.
Cited by
-
The retromer and retriever systems are conserved and differentially expanded in parabasalids.J Cell Sci. 2024 Jul 1;137(13):jcs261949. doi: 10.1242/jcs.261949. Epub 2024 Jul 12. J Cell Sci. 2024. PMID: 38884339 Free PMC article.
-
Mosaic origin of the eukaryotic kinetochore.Proc Natl Acad Sci U S A. 2019 Jun 25;116(26):12873-12882. doi: 10.1073/pnas.1821945116. Epub 2019 May 24. Proc Natl Acad Sci U S A. 2019. PMID: 31127038 Free PMC article.
-
An evolving understanding of sorting signals for endosomal retrieval.iScience. 2022 May 20;25(5):104254. doi: 10.1016/j.isci.2022.104254. Epub 2022 Apr 13. iScience. 2022. PMID: 35434543 Free PMC article. Review.
-
Commander Complex-A Multifaceted Operator in Intracellular Signaling and Cargo.Cells. 2021 Dec 7;10(12):3447. doi: 10.3390/cells10123447. Cells. 2021. PMID: 34943955 Free PMC article. Review.
-
BioPlex Display: An Interactive Suite for Large-Scale AP-MS Protein-Protein Interaction Data.J Proteome Res. 2018 Jan 5;17(1):722-726. doi: 10.1021/acs.jproteome.7b00572. Epub 2017 Oct 31. J Proteome Res. 2018. PMID: 29054129 Free PMC article.
References
-
- Bartuzi P, Hofker MH, van de Sluis B. Tuning NF-kappaB activity: a touch of COMMD proteins. Biochimica et Biophysica Acta. 2013;1832:2315–2321. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

