Nivolumab plus ipilimumab as first-line treatment for advanced non-small-cell lung cancer (CheckMate 012): results of an open-label, phase 1, multicohort study
- PMID: 27932067
- PMCID: PMC5476941
- DOI: 10.1016/S1470-2045(16)30624-6
Nivolumab plus ipilimumab as first-line treatment for advanced non-small-cell lung cancer (CheckMate 012): results of an open-label, phase 1, multicohort study
Abstract
Background: Nivolumab has shown improved survival in the treatment of advanced non-small-cell lung cancer (NSCLC) previously treated with chemotherapy. We assessed the safety and activity of combination nivolumab plus ipilimumab as first-line therapy for NSCLC.
Methods: The open-label, phase 1, multicohort study (CheckMate 012) cohorts reported here were enrolled at eight US academic centres. Eligible patients were aged 18 years or older with histologically or cytologically confirmed recurrent stage IIIb or stage IV, chemotherapy-naive NSCLC. Patients were randomly assigned (1:1:1) by an interactive voice response system to receive nivolumab 1 mg/kg every 2 weeks plus ipilimumab 1 mg/kg every 6 weeks, nivolumab 3 mg/kg every 2 weeks plus ipilimumab 1 mg/kg every 12 weeks, or nivolumab 3 mg/kg every 2 weeks plus ipilimumab 1 mg/kg every 6 weeks until disease progression, unacceptable toxicities, or withdrawal of consent. Data from the latter two cohorts, which were considered potentially suitable for further clinical development, are presented in this report; data from the other cohort (as well as several earlier cohorts) are described in the appendix. The primary outcome was safety and tolerability, assessed in all treated patients. This ongoing study is registered with ClinicalTrials.gov, number NCT01454102.
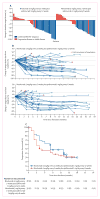
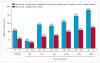
Findings: Between May 15, 2014, and March 25, 2015, 78 patients were randomly assigned to receive nivolumab every 2 weeks plus ipilimumab every 12 weeks (n=38) or nivolumab every 2 weeks plus ipilimumab every 6 weeks (n=40). One patient in the ipilimumab every-6-weeks cohort was excluded before treatment; therefore 77 patients actually received treatment (38 in the ipilimumab every-12-weeks cohort; 39 in the ipilimumab every-6-weeks cohort). At data cut-off on Jan 7, 2016, 29 (76%) patients in the ipilimumab every-12-weeks cohort and 32 (82%) in the ipilimumab every-6-weeks cohort had discontinued treatment. Grade 3-4 treatment-related adverse events occurred in 14 (37%) patients in the ipilimumab every-12-weeks cohort and 13 (33%) patients in the every-6-weeks cohort; the most commonly reported grade 3 or 4 treatment-related adverse events were increased lipase (three [8%] and no patients), pneumonitis (two [5%] and one [3%] patients), adrenal insufficiency (one [3%] and two [5%] patients), and colitis (one [3%] and two [5%] patients). Treatment-related serious adverse events were reported in 12 (32%) patients in the ipilimumab every-12-weeks cohort and 11 (28%) patients in the every-6-weeks cohort. Treatment-related adverse events (any grade) prompted treatment discontinuation in four (11%) patients in the every-12-weeks cohort and five (13%) patients in the every-6-weeks cohort. No treatment-related deaths occurred. Confirmed objective responses were achieved in 18 (47% [95% CI 31-64]) patients in the ipilimumab every-12-weeks cohort and 15 (38% [95% CI 23-55]) patients in the ipilimumab every-6-weeks cohort; median duration of response was not reached in either cohort, with median follow-up times of 12·8 months (IQR 9·3-15·5) in the ipilimumab every-12-weeks cohort and 11·8 months (6·7-15·9) in the ipilimumab every-6-weeks cohort. In patients with PD-L1 of 1% or greater, confirmed objective responses were achieved in 12 (57%) of 21 patients in the ipilimumab every-12-weeks cohort and 13 (57%) of 23 patients in the ipilimumab every-6-weeks cohort.
Interpretation: In NSCLC, first-line nivolumab plus ipilimumab had a tolerable safety profile and showed encouraging clinical activity characterised by a high response rate and durable response. To our knowledge, the results of this study are the first suggestion of improved benefit compared with anti-PD-1 monotherapy in patients with NSCLC, supporting further assessment of this combination in a phase 3 study.
Funding: Bristol-Myers Squibb.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Figures
Comment in
-
Raising the bar on first-line immunotherapy in lung cancer.Lancet Oncol. 2017 Jan;18(1):2-3. doi: 10.1016/S1470-2045(16)30594-0. Epub 2016 Dec 5. Lancet Oncol. 2017. PMID: 27932066 No abstract available.
-
Rushing will not help to choose the best combination.Lancet Oncol. 2017 Apr;18(4):e186. doi: 10.1016/S1470-2045(17)30108-0. Lancet Oncol. 2017. PMID: 28368248 No abstract available.
Similar articles
-
Nivolumab with or without ipilimumab in patients with recurrent or metastatic cervical cancer (CheckMate 358): a phase 1-2, open-label, multicohort trial.Lancet Oncol. 2024 May;25(5):588-602. doi: 10.1016/S1470-2045(24)00088-3. Epub 2024 Apr 9. Lancet Oncol. 2024. PMID: 38608691 Clinical Trial.
-
Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial.Lancet Oncol. 2016 Jul;17(7):883-895. doi: 10.1016/S1470-2045(16)30098-5. Epub 2016 Jun 4. Lancet Oncol. 2016. PMID: 27269741 Clinical Trial.
-
First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial.Lancet Oncol. 2021 Feb;22(2):198-211. doi: 10.1016/S1470-2045(20)30641-0. Epub 2021 Jan 18. Lancet Oncol. 2021. PMID: 33476593 Clinical Trial.
-
Nivolumab plus ipilimumab combination therapy for the first-line treatment NSCLC: evidence to date.Cancer Manag Res. 2019 May 29;11:4893-4904. doi: 10.2147/CMAR.S164935. eCollection 2019. Cancer Manag Res. 2019. PMID: 31213908 Free PMC article. Review.
-
The efficacy and safety of combined immune checkpoint inhibitors (nivolumab plus ipilimumab): a systematic review and meta-analysis.World J Surg Oncol. 2020 Jul 3;18(1):150. doi: 10.1186/s12957-020-01933-5. World J Surg Oncol. 2020. PMID: 32620130 Free PMC article. Review.
Cited by
-
A retrospective study for prognostic significance of type II diabetes mellitus and hemoglobin A1c levels in non-small cell lung cancer patients treated with pembrolizumab.Transl Lung Cancer Res. 2022 Aug;11(8):1619-1630. doi: 10.21037/tlcr-22-493. Transl Lung Cancer Res. 2022. PMID: 36090639 Free PMC article.
-
Research Status and Outlook of PD-1/PD-L1 Inhibitors for Cancer Therapy.Drug Des Devel Ther. 2020 Sep 8;14:3625-3649. doi: 10.2147/DDDT.S267433. eCollection 2020. Drug Des Devel Ther. 2020. PMID: 32982171 Free PMC article. Review.
-
On-Target Side Effects of Targeted Therapeutics of Cancer.Pathol Oncol Res. 2022 Sep 23;28:1610694. doi: 10.3389/pore.2022.1610694. eCollection 2022. Pathol Oncol Res. 2022. PMID: 36213163 Free PMC article. Review.
-
Can Ipilimumab restore immune response in advanced NSCLC after progression on anti-PD-1/PD-L1 agents?Thorac Cancer. 2020 Aug;11(8):2331-2334. doi: 10.1111/1759-7714.13502. Epub 2020 Jun 16. Thorac Cancer. 2020. PMID: 32548905 Free PMC article.
-
Distinct pretreatment innate immune landscape and posttreatment T cell responses underlie immunotherapy-induced colitis.JCI Insight. 2022 Nov 8;7(21):e157839. doi: 10.1172/jci.insight.157839. JCI Insight. 2022. PMID: 36173679 Free PMC article.
References
-
- Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–50. - PubMed
-
- Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26:3543–51. - PubMed
-
- Patel JD, Socinski MA, Garon EB, et al. PointBreak: a randomized phase III study of pemetrexed plus carboplatin and bevacizumab followed by maintenance pemetrexed and bevacizumab versus paclitaxel plus carboplatin and bevacizumab followed by maintenance bevacizumab in patients with stage IIIB or IV nonsquamous non-small-cell lung cancer. J Clin Oncol. 2013;31:4349–57. - PMC - PubMed
-
- Reck M, von Pawel J, Zatloukal P, et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J Clin Oncol. 2009;27:1227–34. - PubMed
-
- Paz-Ares LG, de Marinis F, Dediu M, et al. PARAMOUNT: final overall survival results of the phase III study of maintenance pemetrexed versus placebo immediately after induction treatment with pemetrexed plus cisplatin for advanced nonsquamous non-small-cell lung cancer. J Clin Oncol. 2013;31:2895–902. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

