Association of PD-1/PD-L axis expression with cytolytic activity, mutational load, and prognosis in melanoma and other solid tumors
- PMID: 27837027
- PMCID: PMC5137776
- DOI: 10.1073/pnas.1607836113
Association of PD-1/PD-L axis expression with cytolytic activity, mutational load, and prognosis in melanoma and other solid tumors
Abstract
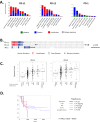
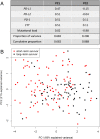
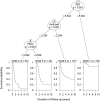
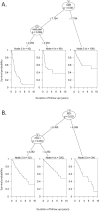
Programmed cell death protein-1 (PD-1)/programmed death ligand-1 (PD-L1) checkpoint blockade has led to remarkable and durable objective responses in a number of different tumor types. A better understanding of factors associated with the PD-1/PD-L axis expression is desirable, as it informs their potential role as prognostic and predictive biomarkers and may suggest rational treatment combinations. In the current study, we analyzed PD-L1, PD-L2, PD-1, and cytolytic activity (CYT) expression, as well as mutational density from melanoma and eight other solid tumor types using The Cancer Genome Atlas database. We found that in some tumor types, PD-L2 expression is more closely linked to Th1/IFNG expression and PD-1 and CD8 signaling than PD-L1 In contrast, mutational load was not correlated with a Th1/IFNG gene signature in any tumor type. PD-L1, PD-L2, PD-1, CYT expression, and mutational density are all positive prognostic features in melanoma, and conditional inference modeling revealed PD-1/CYT expression (i.e., an inflamed tumor microenvironment) as the most impactful feature, followed by mutational density. This study elucidates the highly interdependent nature of these parameters, and also indicates that future biomarkers for anti-PD-1/PD-L1 will benefit from tumor-type-specific, integrated, mRNA, protein, and genomic approaches.
Keywords: PD-1; PD-L1; PD-L2; melanoma; mutational load.
Conflict of interest statement
R.A.A. is a compensated consultant for Adaptive Biotech. D.M.P. has received research grants from Bristol-Myers Squibb and Potenza Therapeutics; consults for Amgen, Five Prime Therapeutics, GlaxoSmithKline, Jounce Therapeutics, MedImmune, Merck, Pfizer, Potenza Therapeutics, and Sanofi; owns stock options in Jounce and Potenza; and receives patent royalties through his institution, from Bristol-Myers Squibb, and Potenza. C.G.D. is a self-compensated consultant for Bristol-Myers Squibb, AstraZeneca/Medimmune, Roche/Genentech, Potenza Therapeutics, and Tizona Therapeutics; and has patents licensed by Bristol-Myers Squibb, AstraZeneca/Medimmune, and Potenza Therapeutics. J.M.T. is a compensated consultant for Bristol-Myers Squibb, AstraZeneca/Medimmune, and Merck.
Figures








Similar articles
-
Quantitative Mass Spectrometry Analysis of PD-L1 Protein Expression, N-glycosylation and Expression Stoichiometry with PD-1 and PD-L2 in Human Melanoma.Mol Cell Proteomics. 2017 Oct;16(10):1705-1717. doi: 10.1074/mcp.RA117.000037. Epub 2017 May 25. Mol Cell Proteomics. 2017. PMID: 28546465 Free PMC article.
-
The expression of programed death ligand-1 could be related with unfavorable prognosis in salivary duct carcinoma.J Oral Pathol Med. 2018 Aug;47(7):683-690. doi: 10.1111/jop.12722. Epub 2018 May 16. J Oral Pathol Med. 2018. PMID: 29719073
-
Prognostic value of programmed death-1, programmed death-ligand 1, programmed death-ligand 2 expression, and CD8(+) T cell density in primary tumors and metastatic lymph nodes from patients with stage T1-4N+M0 gastric adenocarcinoma.Chin J Cancer. 2017 Jul 29;36(1):61. doi: 10.1186/s40880-017-0226-3. Chin J Cancer. 2017. PMID: 28754154 Free PMC article.
-
PD-L1 Expression in Mastocytosis.Int J Mol Sci. 2019 May 13;20(9):2362. doi: 10.3390/ijms20092362. Int J Mol Sci. 2019. PMID: 31086024 Free PMC article. Review.
-
Mechanisms of PD-1/PD-L1 expression and prognostic relevance in non-Hodgkin lymphoma: a summary of immunohistochemical studies.Oncotarget. 2017 Jul 4;8(27):44960-44975. doi: 10.18632/oncotarget.16680. Oncotarget. 2017. PMID: 28402953 Free PMC article. Review.
Cited by
-
Difference in the distribution of tumor-infiltrating CD8+ T cells and FOXP3+ T cells between micronodular thymoma with lymphoid stroma and micronodular thymic carcinoma with lymphoid stroma.Pathol Int. 2021 Jul;71(7):453-462. doi: 10.1111/pin.13099. Epub 2021 Apr 5. Pathol Int. 2021. PMID: 33819365 Free PMC article.
-
In-house Implementation of Tumor Mutational Burden Testing to Predict Durable Clinical Benefit in Non-small Cell Lung Cancer and Melanoma Patients.Cancers (Basel). 2019 Aug 29;11(9):1271. doi: 10.3390/cancers11091271. Cancers (Basel). 2019. PMID: 31470674 Free PMC article.
-
Distinct Molecular Profiles and Immunotherapy Treatment Outcomes of V600E and V600K BRAF-Mutant Melanoma.Clin Cancer Res. 2019 Feb 15;25(4):1272-1279. doi: 10.1158/1078-0432.CCR-18-1680. Epub 2019 Jan 10. Clin Cancer Res. 2019. PMID: 30630828 Free PMC article.
-
Predictive Factors for Response to PD-1/PD-L1 Checkpoint Inhibition in the Field of Hepatocellular Carcinoma: Current Status and Challenges.Cancers (Basel). 2019 Oct 14;11(10):1554. doi: 10.3390/cancers11101554. Cancers (Basel). 2019. PMID: 31615069 Free PMC article. Review.
-
Deciphering Microenvironment of NSCLC based on CD8+ TIL Density and PD-1/PD-L1 Expression.J Cancer. 2019 Jan 1;10(1):211-222. doi: 10.7150/jca.26444. eCollection 2019. J Cancer. 2019. PMID: 30662542 Free PMC article.
References
-
- Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144(5):646–674. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

