The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy
- PMID: 27774485
- PMCID: PMC5045092
- DOI: 10.2147/HP.S93413
The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy
Abstract
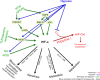
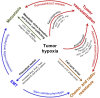
Hypoxia is a non-physiological level of oxygen tension, a phenomenon common in a majority of malignant tumors. Tumor-hypoxia leads to advanced but dysfunctional vascularization and acquisition of epithelial-to-mesenchymal transition phenotype resulting in cell mobility and metastasis. Hypoxia alters cancer cell metabolism and contributes to therapy resistance by inducing cell quiescence. Hypoxia stimulates a complex cell signaling network in cancer cells, including the HIF, PI3K, MAPK, and NFĸB pathways, which interact with each other causing positive and negative feedback loops and enhancing or diminishing hypoxic effects. This review provides background knowledge on the role of tumor hypoxia and the role of the HIF cell signaling involved in tumor blood vessel formation, metastasis, and development of the resistance to therapy. Better understanding of the role of hypoxia in cancer progression will open new windows for the discovery of new therapeutics targeting hypoxic tumor cells and hypoxic microenvironment.
Keywords: angiogenesis; cancer; hypoxia; metastasis; treatment resistance.
Figures
Similar articles
-
Hypoxia-Induced Signaling Promotes Prostate Cancer Progression: Exosomes Role as Messenger of Hypoxic Response in Tumor Microenvironment.Crit Rev Oncog. 2015;20(5-6):419-34. doi: 10.1615/CritRevOncog.v20.i5-6.130. Crit Rev Oncog. 2015. PMID: 27279239 Free PMC article. Review.
-
Solid tumor physiology and hypoxia-induced chemo/radio-resistance: novel strategy for cancer therapy: nitric oxide donor as a therapeutic enhancer.Nitric Oxide. 2008 Sep;19(2):205-16. doi: 10.1016/j.niox.2008.04.026. Epub 2008 May 6. Nitric Oxide. 2008. PMID: 18503779 Review.
-
Hypoxia induced EMT: A review on the mechanism of tumor progression and metastasis in OSCC.Oral Oncol. 2018 May;80:23-32. doi: 10.1016/j.oraloncology.2018.03.004. Epub 2018 Mar 21. Oral Oncol. 2018. PMID: 29706185 Review.
-
Exploiting Current Understanding of Hypoxia Mediated Tumour Progression for Nanotherapeutic Development.Cancers (Basel). 2019 Dec 11;11(12):1989. doi: 10.3390/cancers11121989. Cancers (Basel). 2019. PMID: 31835751 Free PMC article. Review.
-
HIF-1α promoted vasculogenic mimicry formation in hepatocellular carcinoma through LOXL2 up-regulation in hypoxic tumor microenvironment.J Exp Clin Cancer Res. 2017 Apr 27;36(1):60. doi: 10.1186/s13046-017-0533-1. J Exp Clin Cancer Res. 2017. PMID: 28449718 Free PMC article.
Cited by
-
Aging of the progenitor cells that initiate prostate cancer.Cancer Lett. 2021 Sep 1;515:28-35. doi: 10.1016/j.canlet.2021.05.014. Epub 2021 May 28. Cancer Lett. 2021. PMID: 34052326 Free PMC article. Review.
-
Histone variant H2AZ1 drives lung cancer progression through the RELA-HIF1A-EGFR signaling pathway.Cell Commun Signal. 2024 Sep 26;22(1):453. doi: 10.1186/s12964-024-01823-3. Cell Commun Signal. 2024. PMID: 39327549 Free PMC article.
-
Loss of Fer Jeopardizes Metabolic Plasticity and Mitochondrial Homeostasis in Lung and Breast Carcinoma Cells.Int J Mol Sci. 2021 Mar 25;22(7):3387. doi: 10.3390/ijms22073387. Int J Mol Sci. 2021. PMID: 33806191 Free PMC article.
-
Neuromodulation Applied to Diseases: The Case of HRV Biofeedback.J Clin Med. 2022 Oct 8;11(19):5927. doi: 10.3390/jcm11195927. J Clin Med. 2022. PMID: 36233794 Free PMC article. Review.
-
Bioresponsive and immunotherapeutic nanomaterials to remodel tumor microenvironment for enhanced immune checkpoint blockade.Bioact Mater. 2023 Nov 3;32:530-542. doi: 10.1016/j.bioactmat.2023.10.023. eCollection 2024 Feb. Bioact Mater. 2023. PMID: 38026439 Free PMC article.
References
-
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. - PubMed
-
- Semenza GL. Hypoxia, clonal selection, and the role of HIF-1 in tumor progression. Crit Rev Biochem Mol Biol. 2000;35(2):71–103. - PubMed
-
- Hockel M, Schlenger K, Knoop C, Vaupel P. Oxygenation of carcinomas of the uterine cervix: evaluation by computerized O2 tension measurements. Cancer Res. 1991;51(22):6098–6102. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

