A Phase 1 Study of 4 Live, Recombinant Human Cytomegalovirus Towne/Toledo Chimera Vaccines in Cytomegalovirus-Seronegative Men
- PMID: 27521362
- PMCID: PMC5079366
- DOI: 10.1093/infdis/jiw365
A Phase 1 Study of 4 Live, Recombinant Human Cytomegalovirus Towne/Toledo Chimera Vaccines in Cytomegalovirus-Seronegative Men
Abstract
Background: Human cytomegalovirus (HCMV) infection causes disease in newborns and transplant recipients. A HCMV vaccine (Towne) protects transplant recipients.
Methods: The genomes of Towne and the nonattenuated Toledo strain were recombined, yielding 4 Towne/Toledo chimera vaccines. Each of 36 HCMV-seronegative men received 1 subcutaneous dose of 10, 100, or 1000 plaque-forming units (PFU) in cohorts of 3. Safety and immunogenicity were evaluated over 12 weeks after immunization and for 52 weeks for those who seroconverted.
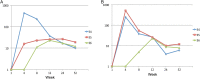
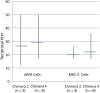
Results: There were no serious local or systemic reactions. No subject had HCMV in urine or saliva. For chimera 3, none of 9 subjects seroconverted. For chimera 1, 1 of 9 seroconverted (the seroconverter received 100 PFU). For chimera 2, 3 subjects seroconverted (1 received 100 PFU, and 2 received 1000 PFU). For chimera 4, 7 subjects seroconverted (1 received 10 PFU, 3 received 100 PFU, and 3 received 1000 PFU). All 11 seroconverters developed low but detectable levels of neutralizing activity. CD4+ T-cell responses were detectable in 1 subject (who received 100 PFU of chimera 4). Seven subjects receiving chimera 2 or 4 had detectable CD8+ T-cell responses to IE1; 3 responded to 1-2 additional antigens.
Conclusions: The Towne/Toledo chimera vaccine candidates were well tolerated and were not excreted. Additional human trials of chimeras 2 and 4 are appropriate.
Clinical trials registration: NCT01195571.
Keywords: cytomegalovirus; pregnancy; transplantation; vaccines.
© The Author 2016. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail journals.permissions@oup.com.
Figures

Comment in
-
40 Years Is Long Enough!J Infect Dis. 2016 Nov 1;214(9):1297-1299. doi: 10.1093/infdis/jiw367. Epub 2016 Aug 11. J Infect Dis. 2016. PMID: 27521360 No abstract available.
Similar articles
-
A phase 1 study of 4 live, recombinant human cytomegalovirus Towne/Toledo chimeric vaccines.J Infect Dis. 2006 May 15;193(10):1350-60. doi: 10.1086/503365. Epub 2006 Apr 12. J Infect Dis. 2006. PMID: 16619181 Clinical Trial.
-
Comparative virulence and immunogenicity of the Towne strain and a nonattenuated strain of cytomegalovirus.Ann Intern Med. 1984 Oct;101(4):478-83. doi: 10.7326/0003-4819-101-4-478. Ann Intern Med. 1984. PMID: 6089634
-
Genomic analysis of chimeric human cytomegalovirus vaccine candidates derived from strains Towne and Toledo.Virus Genes. 2017 Aug;53(4):650-655. doi: 10.1007/s11262-017-1452-0. Epub 2017 Apr 8. Virus Genes. 2017. PMID: 28391502 Free PMC article.
-
Human cytomegalovirus (HCMV) infection/re-infection: development of a protective HCMV vaccine.New Microbiol. 2019 Jan;42(1):1-20. Epub 2019 Jan 21. New Microbiol. 2019. PMID: 30671581 Review.
-
Development of a cytomegalovirus vaccine: lessons from recent clinical trials.Expert Opin Biol Ther. 2001 May;1(3):401-12. doi: 10.1517/14712598.1.3.401. Expert Opin Biol Ther. 2001. PMID: 11727514 Review.
Cited by
-
Congenital Cytomegalovirus: a "Now" Problem-No Really, Now.Clin Vaccine Immunol. 2017 Jan 5;24(1):e00491-16. doi: 10.1128/CVI.00491-16. Print 2017 Jan. Clin Vaccine Immunol. 2017. PMID: 27795304 Free PMC article.
-
Advances in the treatment of cytomegalovirus.Br Med Bull. 2019 Sep 19;131(1):5-17. doi: 10.1093/bmb/ldz031. Br Med Bull. 2019. PMID: 31580403 Free PMC article.
-
SMAC mimetics inhibit human T cell proliferation and fail to augment type 1 cytokine responses.Cell Immunol. 2023 Feb;384:104674. doi: 10.1016/j.cellimm.2023.104674. Epub 2023 Jan 18. Cell Immunol. 2023. PMID: 36706656 Free PMC article.
-
Where do we Stand after Decades of Studying Human Cytomegalovirus?Microorganisms. 2020 May 8;8(5):685. doi: 10.3390/microorganisms8050685. Microorganisms. 2020. PMID: 32397070 Free PMC article. Review.
-
Towards a Prophylactic Vaccine for the Prevention of HCMV Infection.Vaccines (Basel). 2021 Aug 29;9(9):968. doi: 10.3390/vaccines9090968. Vaccines (Basel). 2021. PMID: 34579205 Free PMC article.
References
-
- Fowler KB, Stagno S, Pass RF, Britt WJ, Boll TJ, Alford CA. Outcome of congenital cytomegalovirus infection in relation to maternal antibody status. N Engl J Med 1992; 326:663–7. - PubMed
-
- Stratton KR, Durch J, Lawrence RS. Vaccines for the 21st century: a tool for decision making. Washington, DC: National Academy Press, 2000. - PubMed
-
- Plotkin S. The history of vaccination against cytomegalovirus. Med Microbiol Immunol 2015; 204:247–54. - PubMed
-
- Elek SD, Stern H. Development of a vaccine against mental retardation cased by cytomegalovirus infection in utero. Lancet 1974; 1:1–15. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

