Chlamydia cell biology and pathogenesis
- PMID: 27108705
- PMCID: PMC4886739
- DOI: 10.1038/nrmicro.2016.30
Chlamydia cell biology and pathogenesis
Abstract
Chlamydia spp. are important causes of human disease for which no effective vaccine exists. These obligate intracellular pathogens replicate in a specialized membrane compartment and use a large arsenal of secreted effectors to survive in the hostile intracellular environment of the host. In this Review, we summarize the progress in decoding the interactions between Chlamydia spp. and their hosts that has been made possible by recent technological advances in chlamydial proteomics and genetics. The field is now poised to decipher the molecular mechanisms that underlie the intimate interactions between Chlamydia spp. and their hosts, which will open up many exciting avenues of research for these medically important pathogens.
Figures
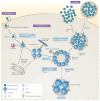

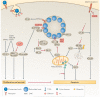
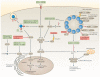
Similar articles
-
Effector protein modulation of host cells: examples in the Chlamydia spp. arsenal.Curr Opin Microbiol. 2009 Feb;12(1):81-7. doi: 10.1016/j.mib.2008.11.009. Epub 2009 Jan 8. Curr Opin Microbiol. 2009. PMID: 19138553 Review.
-
A meta-analysis of affinity purification-mass spectrometry experimental systems used to identify eukaryotic and chlamydial proteins at the Chlamydia trachomatis inclusion membrane.J Proteomics. 2020 Feb 10;212:103595. doi: 10.1016/j.jprot.2019.103595. Epub 2019 Nov 21. J Proteomics. 2020. PMID: 31760040 Free PMC article.
-
Subversion of Cell-Autonomous Host Defense by Chlamydia Infection.Curr Top Microbiol Immunol. 2018;412:81-106. doi: 10.1007/82_2016_13. Curr Top Microbiol Immunol. 2018. PMID: 27169422 Review.
-
Chlamydial intracellular survival strategies.Cold Spring Harb Perspect Med. 2013 May 1;3(5):a010256. doi: 10.1101/cshperspect.a010256. Cold Spring Harb Perspect Med. 2013. PMID: 23637308 Free PMC article. Review.
-
Got mutants? How advances in chlamydial genetics have furthered the study of effector proteins.Pathog Dis. 2021 Feb 4;79(2):ftaa078. doi: 10.1093/femspd/ftaa078. Pathog Dis. 2021. PMID: 33512479 Free PMC article. Review.
Cited by
-
Insights Into Host Cell Cytokines in Chlamydia Infection.Front Immunol. 2021 May 21;12:639834. doi: 10.3389/fimmu.2021.639834. eCollection 2021. Front Immunol. 2021. PMID: 34093528 Free PMC article. Review.
-
Chlamydia repurposes the actin-binding protein EPS8 to disassemble epithelial tight junctions and promote infection.Cell Host Microbe. 2022 Dec 14;30(12):1685-1700.e10. doi: 10.1016/j.chom.2022.10.013. Epub 2022 Nov 16. Cell Host Microbe. 2022. PMID: 36395759 Free PMC article.
-
Application of a C. trachomatis expression system to identify C. pneumoniae proteins translocated into host cells.J Bacteriol. 2021 Jun 1;203(11):e00511-20. doi: 10.1128/JB.00511-20. Epub 2021 Mar 8. J Bacteriol. 2021. PMID: 33685970 Free PMC article.
-
Expression activation of over 70% of Chlamydia trachomatis genes during the first hour of infection.Infect Immun. 2024 Mar 12;92(3):e0053923. doi: 10.1128/iai.00539-23. Epub 2024 Feb 1. Infect Immun. 2024. PMID: 38299827 Free PMC article.
-
A predation assay using amoebae to screen for virulence factors unearthed the first W. chondrophila inclusion membrane protein.Sci Rep. 2019 Dec 20;9(1):19485. doi: 10.1038/s41598-019-55511-1. Sci Rep. 2019. PMID: 31862969 Free PMC article.
References
-
- Bachmann NL, Polkinghorne A, Timms P. Chlamydia genomics: providing novel insights into chlamydial biology. Trends Microbiol. 2014;22:464–472. - PubMed
-
- Mandell GL, Bennett JE, Dolin R, editors. Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases. 7th edn Vol. 2. Churchill Livingstone/Elsevier; 2010.
-
- Rank RG. In: Intracellular Pathogens 1: Chlamydiales. Tan M, Bavoil PM, editors. Vol. 1. ASM Press; 2012. pp. 285–310.
-
- de Vrieze NH, de Vries HJ. Lymphogranuloma venereum among men who have sex with men. An epidemiological and clinical review. Expert Rev. Anti Infect. Ther. 2014;12:697–704. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

