Afatinib versus methotrexate in older patients with second-line recurrent and/or metastatic head and neck squamous cell carcinoma: subgroup analysis of the LUX-Head & Neck 1 trial
- PMID: 27084954
- PMCID: PMC4959921
- DOI: 10.1093/annonc/mdw151
Afatinib versus methotrexate in older patients with second-line recurrent and/or metastatic head and neck squamous cell carcinoma: subgroup analysis of the LUX-Head & Neck 1 trial
Abstract
Background: In the phase III LUX-Head & Neck 1 (LHN1) trial, afatinib significantly improved progression-free survival (PFS) versus methotrexate in recurrent and/or metastatic (R/M) head and neck squamous cell carcinoma (HNSCC) patients progressing on/after platinum-based therapy. This report evaluates afatinib efficacy and safety in prespecified subgroups of patients aged ≥65 and <65 years.
Patients and methods: Patients were randomized (2:1) to 40 mg/day oral afatinib or 40 mg/m(2)/week intravenous methotrexate. PFS was the primary end point; overall survival (OS) was the key secondary end point. Other end points included: objective response rate (ORR), patient-reported outcomes, tumor shrinkage, and safety. Disease control rate (DCR) was also assessed.
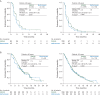
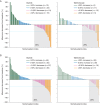
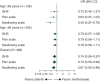
Results: Of 483 randomized patients, 27% (83 afatinib; 45 methotrexate) were aged ≥65 years (older) and 73% (239 afatinib; 116 methotrexate) <65 years (younger) at study entry. Similar PFS benefit with afatinib versus methotrexate was observed in older {median 2.8 versus 2.3 months, hazard ratio (HR) = 0.68 [95% confidence interval (CI) 0.45-1.03], P = 0.061} and younger patients [2.6 versus 1.6 months, HR = 0.79 (0.62-1.01), P = 0.052]. In older and younger patients, the median OS with afatinib versus methotrexate was 7.3 versus 6.4 months [HR = 0.84 (0.54-1.31)] and 6.7 versus 6.2 months [HR = 0.98 (0.76-1.28)]. ORRs with afatinib versus methotrexate were 10.8% versus 6.7% and 10.0% versus 5.2%; DCRs were 53.0% versus 37.8% and 47.7% versus 38.8% in older and younger patients, respectively. In both subgroups, the most frequent treatment-related adverse events were rash/acne (73%-77%) and diarrhea (70%-80%) with afatinib, and stomatitis (43%) and fatigue (31%-34%) with methotrexate. Fewer treatment-related discontinuations were observed with afatinib (each subgroup 7% versus 16%). A trend toward improved time to deterioration of global health status, pain, and swallowing with afatinib was observed in both subgroups.
Conclusions: Advancing age (≥65 years) did not adversely affect clinical outcomes or safety with afatinib versus methotrexate in second-line R/M HNSCC patients.
Clinical trial registration: NCT01345682 (ClinicalTrials.gov).
Keywords: HNSCC; afatinib; methotrexate; older; phase III; second-line.
© The Author 2016. Published by Oxford University Press on behalf of the European Society for Medical Oncology.
Figures
Similar articles
-
Afatinib versus methotrexate as second-line treatment in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck progressing on or after platinum-based therapy (LUX-Head & Neck 1): an open-label, randomised phase 3 trial.Lancet Oncol. 2015 May;16(5):583-94. doi: 10.1016/S1470-2045(15)70124-5. Epub 2015 Apr 16. Lancet Oncol. 2015. PMID: 25892145 Clinical Trial.
-
Biomarkers predict enhanced clinical outcomes with afatinib versus methotrexate in patients with second-line recurrent and/or metastatic head and neck cancer.Ann Oncol. 2017 Oct 1;28(10):2526-2532. doi: 10.1093/annonc/mdx344. Ann Oncol. 2017. PMID: 28961833 Free PMC article. Clinical Trial.
-
Rationale and design of LUX-Head & Neck 1: a randomised, Phase III trial of afatinib versus methotrexate in patients with recurrent and/or metastatic head and neck squamous cell carcinoma who progressed after platinum-based therapy.BMC Cancer. 2014 Jun 28;14:473. doi: 10.1186/1471-2407-14-473. BMC Cancer. 2014. PMID: 24973959 Free PMC article. Clinical Trial.
-
Afatinib in squamous cell carcinoma of the head and neck.Expert Opin Pharmacother. 2016 Jun;17(9):1295-301. doi: 10.1080/14656566.2016.1183647. Epub 2016 May 19. Expert Opin Pharmacother. 2016. PMID: 27160335 Review.
-
Afatinib in the treatment of head and neck squamous cell carcinoma.Expert Opin Investig Drugs. 2014 Jan;23(1):135-43. doi: 10.1517/13543784.2014.858696. Epub 2013 Nov 25. Expert Opin Investig Drugs. 2014. PMID: 24266694 Review.
Cited by
-
Treatment of Elderly Patients with Squamous Cell Carcinoma of the Head and Neck.Front Oncol. 2016 Aug 31;6:199. doi: 10.3389/fonc.2016.00199. eCollection 2016. Front Oncol. 2016. PMID: 27630826 Free PMC article. Review.
-
Efficacy of Afatinib in the Treatment of Patients with Non-Small Cell Lung Cancer and Head and Neck Squamous Cell Carcinoma: A Systematic Review and Meta-Analysis.Cancers (Basel). 2021 Feb 8;13(4):688. doi: 10.3390/cancers13040688. Cancers (Basel). 2021. PMID: 33567737 Free PMC article. Review.
-
Appropriate Sequence for Afatinib and Cisplatin Combination Improves Anticancer Activity in Head and Neck Squamous Cell Carcinoma.Front Oncol. 2018 Oct 5;8:432. doi: 10.3389/fonc.2018.00432. eCollection 2018. Front Oncol. 2018. PMID: 30345256 Free PMC article.
-
Exploring the Therapeutic Implications of Co-Targeting the EGFR and Spindle Assembly Checkpoint Pathways in Oral Cancer.Pharmaceutics. 2024 Sep 11;16(9):1196. doi: 10.3390/pharmaceutics16091196. Pharmaceutics. 2024. PMID: 39339232 Free PMC article. Review.
-
Systemic Drug-induced Chronic Paronychia and Periungual Pyogenic Granuloma.Indian Dermatol Online J. 2018 Sep-Oct;9(5):293-298. doi: 10.4103/idoj.IDOJ_133_18. Indian Dermatol Online J. 2018. PMID: 30258794 Free PMC article. Review.
References
-
- Ferlay J, Soerjomataram I, Ervik M et al. . GLOBOCAN 2012 v1.0: Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11. 2012.
-
- Gatta G, Botta L, Sanchez MJ et al. . Prognoses and improvement for head and neck cancers diagnosed in Europe in early 2000s: The EUROCARE-5 population-based study. Eur J Cancer 2015; 51: 2130–2143. - PubMed
-
- Sarris EG, Harrington KJ, Saif MW, Syrigos KN. Multimodal treatment strategies for elderly patients with head and neck cancer. Cancer Treat Rev 2014; 40: 465–475. - PubMed
-
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Head and Neck Cancers, Version 1.2015. 2015. In http://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf (11 March 2016, date last accessed). - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

