Extracellular Vesicles Exploit Viral Entry Routes for Cargo Delivery
- PMID: 26935137
- PMCID: PMC4867369
- DOI: 10.1128/MMBR.00063-15
Extracellular Vesicles Exploit Viral Entry Routes for Cargo Delivery
Abstract
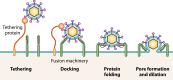
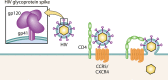
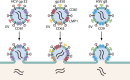
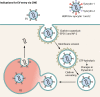
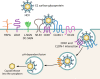
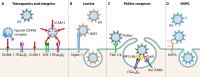
Extracellular vesicles (EVs) have emerged as crucial mediators of intercellular communication, being involved in a wide array of key biological processes. Eukaryotic cells, and also bacteria, actively release heterogeneous subtypes of EVs into the extracellular space, where their contents reflect their (sub)cellular origin and the physiologic state of the parent cell. Within the past 20 years, presumed subtypes of EVs have been given a rather confusing diversity of names, including exosomes, microvesicles, ectosomes, microparticles, virosomes, virus-like particles, and oncosomes, and these names are variously defined by biogenesis, physical characteristics, or function. The latter category, functions, in particular the transmission of biological signals between cells in vivo and how EVs control biological processes, has garnered much interest. EVs have pathophysiological properties in cancer, neurodegenerative disorders, infectious disease, and cardiovascular disease, highlighting possibilities not only for minimally invasive diagnostic applications but also for therapeutic interventions, like macromolecular drug delivery. Yet, in order to pursue therapies involving EVs and delivering their cargo, a better grasp of EV targeting is needed. Here, we review recent progress in understanding the molecular mechanisms underpinning EV uptake by receptor-ligand interactions with recipient cells, highlighting once again the overlap of EVs and viruses. Despite their highly heterogeneous nature, EVs require common viral entry pathways, and an unanticipated specificity for cargo delivery is being revealed. We discuss the challenges ahead in delineating specific roles for EV-associated ligands and cellular receptors.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures





Similar articles
-
Translating extracellular vesicle packaging into therapeutic applications.Front Immunol. 2022 Aug 15;13:946422. doi: 10.3389/fimmu.2022.946422. eCollection 2022. Front Immunol. 2022. PMID: 36045692 Free PMC article. Review.
-
Extracellular vesicles (EVs)' journey in recipient cells: from recognition to cargo release.J Zhejiang Univ Sci B. 2024 Aug 15;25(8):633-655. doi: 10.1631/jzus.B2300566. J Zhejiang Univ Sci B. 2024. PMID: 39155778 Free PMC article. Review.
-
Biogenesis of Extracellular Vesicles.Subcell Biochem. 2021;97:19-43. doi: 10.1007/978-3-030-67171-6_2. Subcell Biochem. 2021. PMID: 33779912
-
Intercellular Molecular Transfer Mediated by Extracellular Vesicles in Cancer.Results Probl Cell Differ. 2024;73:327-352. doi: 10.1007/978-3-031-62036-2_14. Results Probl Cell Differ. 2024. PMID: 39242385 Review.
-
Introduction to the Community of Extracellular Vesicles.Subcell Biochem. 2021;97:3-18. doi: 10.1007/978-3-030-67171-6_1. Subcell Biochem. 2021. PMID: 33779911
Cited by
-
Cationized extracellular vesicles for gene delivery.Sci Rep. 2024 Oct 28;14(1):25818. doi: 10.1038/s41598-024-75985-y. Sci Rep. 2024. PMID: 39468145 Free PMC article.
-
The balance between helper T 17 and regulatory T cells in osteoimmunology and relevant research progress on bone tissue engineering.Immun Inflamm Dis. 2024 Sep;12(9):e70011. doi: 10.1002/iid3.70011. Immun Inflamm Dis. 2024. PMID: 39264247 Free PMC article. Review.
-
Engineered extracellular vesicle-delivered TGF-β inhibitor for attenuating osteoarthritis by targeting subchondral bone.J Tissue Eng. 2024 Jul 24;15:20417314241257781. doi: 10.1177/20417314241257781. eCollection 2024 Jan-Dec. J Tissue Eng. 2024. PMID: 39071897 Free PMC article.
-
Zika Virus-Infected Monocyte Exosomes Mediate Cell-to-Cell Viral Transmission.Cells. 2024 Jan 12;13(2):144. doi: 10.3390/cells13020144. Cells. 2024. PMID: 38247836 Free PMC article.
-
Lipid Metabolism Modulation during SARS-CoV-2 Infection: A Spotlight on Extracellular Vesicles and Therapeutic Prospects.Int J Mol Sci. 2024 Jan 4;25(1):640. doi: 10.3390/ijms25010640. Int J Mol Sci. 2024. PMID: 38203811 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

