Distinct regulatory mechanisms governing embryonic versus adult adipocyte maturation
- PMID: 26280538
- PMCID: PMC4553131
- DOI: 10.1038/ncb3217
Distinct regulatory mechanisms governing embryonic versus adult adipocyte maturation
Abstract
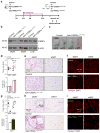
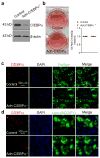
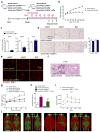
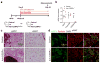
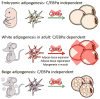
Pathological expansion of adipose tissue contributes to the metabolic syndrome. Distinct depots develop at various times under different physiological conditions. The transcriptional cascade mediating adipogenesis is established in vitro, and centres around a core program involving PPARγ and C/EBPα. We developed an inducible, adipocyte-specific knockout system to probe the requirement of key adipogenic transcription factors at various stages of adipogenesis in vivo. C/EBPα is essential for all white adipogenic conditions in the adult stage, such as adipose tissue regeneration, adipogenesis in muscle and unhealthy expansion of white adipose tissue during high-fat feeding or due to leptin deficiency. Surprisingly, terminal embryonic adipogenesis is fully C/EBPα independent, but does however depend on PPARγ; cold-induced beige adipogenesis is also C/EBPα independent. Moreover, C/EBPα is not vital for adipocyte survival in the adult stage. We reveal a surprising diversity of transcriptional signals required at different stages of adipogenesis in vivo.
Figures



Similar articles
-
Pdcd4 restrains the self-renewal and white-to-beige transdifferentiation of adipose-derived stem cells.Cell Death Dis. 2016 Mar 31;7(3):e2169. doi: 10.1038/cddis.2016.75. Cell Death Dis. 2016. PMID: 27031966 Free PMC article.
-
Role of C/EBPβ-LAP and C/EBPβ-LIP in early adipogenic differentiation of human white adipose-derived progenitors and at later stages in immature adipocytes.Differentiation. 2013 Jan;85(1-2):20-31. doi: 10.1016/j.diff.2012.11.001. Epub 2013 Jan 11. Differentiation. 2013. PMID: 23314288
-
Alpinia officinarum inhibits adipocyte differentiation and high-fat diet-induced obesity in mice through regulation of adipogenesis and lipogenesis.J Med Food. 2012 Nov;15(11):959-67. doi: 10.1089/jmf.2012.2286. J Med Food. 2012. PMID: 23126661
-
Transcriptional Regulation of Adipogenesis.Compr Physiol. 2017 Mar 16;7(2):635-674. doi: 10.1002/cphy.c160022. Compr Physiol. 2017. PMID: 28333384 Review.
-
Molecular Regulation of Adipogenesis and Potential Anti-Adipogenic Bioactive Molecules.Int J Mol Sci. 2016 Jan 19;17(1):124. doi: 10.3390/ijms17010124. Int J Mol Sci. 2016. PMID: 26797605 Free PMC article. Review.
Cited by
-
Perivascular Adipose Tissue and Perivascular Adipose Tissue-Derived Extracellular Vesicles: New Insights in Vascular Disease.Cells. 2024 Aug 6;13(16):1309. doi: 10.3390/cells13161309. Cells. 2024. PMID: 39195199 Free PMC article. Review.
-
Transcriptome and Metabolome Analyses Provide Insight into the Glucose-Induced Adipogenesis in Porcine Adipocytes.Curr Issues Mol Biol. 2024 Mar 3;46(3):2027-2042. doi: 10.3390/cimb46030131. Curr Issues Mol Biol. 2024. PMID: 38534747 Free PMC article.
-
The Impact of Maternal Obesity on Adipose Progenitor Cells.Biomedicines. 2023 Dec 8;11(12):3252. doi: 10.3390/biomedicines11123252. Biomedicines. 2023. PMID: 38137473 Free PMC article. Review.
-
Differential roles of insulin receptor in adipocyte progenitor cells in mice.Mol Cell Endocrinol. 2023 Aug 1;573:111968. doi: 10.1016/j.mce.2023.111968. Epub 2023 May 25. Mol Cell Endocrinol. 2023. PMID: 37244600 Free PMC article.
-
Characteristic and fate determination of adipose precursors during adipose tissue remodeling.Cell Regen. 2023 May 4;12(1):13. doi: 10.1186/s13619-023-00157-8. Cell Regen. 2023. PMID: 37138165 Free PMC article. Review.
References
-
- Scherer PE. Adipose tissue: from lipid storage compartment to endocrine organ. Diabetes. 2006;55:1537–1545. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

