Identification and optimization of 2-aminobenzimidazole derivatives as novel inhibitors of TRPC4 and TRPC5 channels
- PMID: 25816897
- PMCID: PMC4507155
- DOI: 10.1111/bph.13140
Identification and optimization of 2-aminobenzimidazole derivatives as novel inhibitors of TRPC4 and TRPC5 channels
Abstract
Background and purpose: Transient receptor potential canonical (TRPC) channels play important roles in a broad array of physiological functions and are involved in various diseases. However, due to a lack of potent subtype-specific inhibitors the exact roles of TRPC channels in physiological and pathophysiological conditions have not been elucidated.
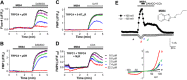
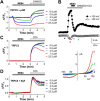
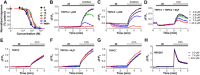
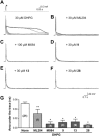
Experimental approach: Using fluorescence membrane potential and Ca(2+) assays and electrophysiological recordings, we characterized new 2-aminobenzimidazole-based small molecule inhibitors of TRPC4 and TRPC5 channels identified from cell-based fluorescence high-throughput screening.
Key results: The original compound, M084, was a potent inhibitor of both TRPC4 and TRPC5, but was also a weak inhibitor of TRPC3. Structural modifications of the lead compound resulted in the identification of analogues with improved potency and selectivity for TRPC4 and TRPC5 channels. The aminobenzimidazole derivatives rapidly inhibited the TRPC4- and TRPC5-mediated currents when applied from the extracellular side and this inhibition was independent of the mode of activation of these channels. The compounds effectively blocked the plateau potential mediated by TRPC4-containing channels in mouse lateral septal neurons, but did not affect the activity of heterologously expressed TRPA1, TRPM8, TRPV1 or TRPV3 channels or that of the native voltage-gated Na(+) , K(+) and Ca(2) (+) channels in dissociated neurons.
Conclusions and implications: The TRPC4/C5-selective inhibitors developed here represent novel and useful pharmaceutical tools for investigation of physiological and pathophysiological functions of TRPC4/C5 channels.
© 2015 The British Pharmacological Society.
Figures



Similar articles
-
Identification of ML204, a novel potent antagonist that selectively modulates native TRPC4/C5 ion channels.J Biol Chem. 2011 Sep 23;286(38):33436-46. doi: 10.1074/jbc.M111.274167. Epub 2011 Jul 27. J Biol Chem. 2011. PMID: 21795696 Free PMC article.
-
Potent, selective, and subunit-dependent activation of TRPC5 channels by a xanthine derivative.Br J Pharmacol. 2019 Oct;176(20):3924-3938. doi: 10.1111/bph.14791. Epub 2019 Sep 6. Br J Pharmacol. 2019. PMID: 31277085 Free PMC article.
-
Effect of non-steroidal anti-inflammatory drugs and new fenamate analogues on TRPC4 and TRPC5 channels.Biochem Pharmacol. 2012 Apr 1;83(7):923-31. doi: 10.1016/j.bcp.2012.01.014. Epub 2012 Jan 20. Biochem Pharmacol. 2012. PMID: 22285229
-
TRPC4- and TRPC4-containing channels.Handb Exp Pharmacol. 2014;222:85-128. doi: 10.1007/978-3-642-54215-2_5. Handb Exp Pharmacol. 2014. PMID: 24756704 Review.
-
TRPC1 as a negative regulator for TRPC4 and TRPC5 channels.Pflugers Arch. 2019 Aug;471(8):1045-1053. doi: 10.1007/s00424-019-02289-w. Epub 2019 Jun 20. Pflugers Arch. 2019. PMID: 31222490 Review.
Cited by
-
Involvement of TRPC5 channels, inwardly rectifying K+ channels, PLCβ and PIP2 in vasopressin-mediated excitation of medial central amygdala neurons.J Physiol. 2021 Jun;599(12):3101-3119. doi: 10.1113/JP281260. Epub 2021 Apr 27. J Physiol. 2021. PMID: 33871877 Free PMC article.
-
TRP Channels as Drug Targets to Relieve Itch.Pharmaceuticals (Basel). 2018 Oct 6;11(4):100. doi: 10.3390/ph11040100. Pharmaceuticals (Basel). 2018. PMID: 30301231 Free PMC article. Review.
-
Treasure troves of pharmacological tools to study transient receptor potential canonical 1/4/5 channels.Br J Pharmacol. 2019 Apr;176(7):832-846. doi: 10.1111/bph.14578. Epub 2019 Mar 6. Br J Pharmacol. 2019. PMID: 30656647 Free PMC article. Review.
-
Endothelial TRPV1 as an Emerging Molecular Target to Promote Therapeutic Angiogenesis.Cells. 2020 May 27;9(6):1341. doi: 10.3390/cells9061341. Cells. 2020. PMID: 32471282 Free PMC article. Review.
-
Benzimidazole derivative M084 extends the lifespan of Caenorhabditis elegans in a DAF-16/FOXO-dependent way.Mol Cell Biochem. 2017 Feb;426(1-2):101-109. doi: 10.1007/s11010-016-2884-x. Epub 2016 Nov 16. Mol Cell Biochem. 2017. PMID: 27854075
References
-
- Antolín AA, Mestres J. Distant polypharmacology among MLP chemical probes. ACS Chem Biol. 2015;10:395–400. - PubMed
-
- Calvo RR, Meegalla SK, Parks DJ, Parsons WH, Ballentine SK, Lubin ML, et al. Discovery of vinylcycloalkyl-substituted benzimidazole TRPM8 antagonists effective in the treatment of cold allodynia. Bioorg Med Chem Lett. 2012;22:1903–1907. - PubMed
-
- Eder P, Poteser M, Romanin C, Groschner K. Na+ entry and modulation of Na+/Ca2+ exchange as a key mechanism of TRPC signaling. Pflugers Arch. 2005;451:99–104. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

