(-)-Englerin A is a potent and selective activator of TRPC4 and TRPC5 calcium channels
- PMID: 25707820
- PMCID: PMC7116557
- DOI: 10.1002/anie.201411511
(-)-Englerin A is a potent and selective activator of TRPC4 and TRPC5 calcium channels
Abstract
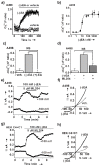
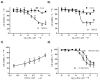
Current therapies for common types of cancer such as renal cell cancer are often ineffective and unspecific, and novel pharmacological targets and approaches are in high demand. Here we show the unexpected possibility for the rapid and selective killing of renal cancer cells through activation of calcium-permeable nonselective transient receptor potential canonical (TRPC) calcium channels by the sesquiterpene (-)-englerin A. This compound was found to be a highly efficient, fast-acting, potent, selective, and direct stimulator of TRPC4 and TRPC5 channels. TRPC4/5 activation through a high-affinity extracellular (-)-englerin A binding site may open up novel opportunities for drug discovery aimed at renal cancer.
Keywords: antitumor agents; calcium ions; ion channels; natural products.
© 2015 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures

Similar articles
-
Englerin A Agonizes the TRPC4/C5 Cation Channels to Inhibit Tumor Cell Line Proliferation.PLoS One. 2015 Jun 22;10(6):e0127498. doi: 10.1371/journal.pone.0127498. eCollection 2015. PLoS One. 2015. PMID: 26098886 Free PMC article.
-
Identification of an (-)-englerin A analogue, which antagonizes (-)-englerin A at TRPC1/4/5 channels.Br J Pharmacol. 2018 Mar;175(5):830-839. doi: 10.1111/bph.14128. Epub 2018 Jan 25. Br J Pharmacol. 2018. PMID: 29247460 Free PMC article.
-
Shape Similarity by Fractal Dimensionality: An Application in the de novo Design of (-)-Englerin A Mimetics.ChemMedChem. 2020 Apr 3;15(7):566-570. doi: 10.1002/cmdc.202000017. Epub 2020 Mar 12. ChemMedChem. 2020. PMID: 32162837
-
TRPC4 and TRPC5: receptor-operated Ca2+-permeable nonselective cation channels.Cell Calcium. 2003 May-Jun;33(5-6):441-50. doi: 10.1016/s0143-4160(03)00055-1. Cell Calcium. 2003. PMID: 12765689 Review.
-
TRPC1 as a negative regulator for TRPC4 and TRPC5 channels.Pflugers Arch. 2019 Aug;471(8):1045-1053. doi: 10.1007/s00424-019-02289-w. Epub 2019 Jun 20. Pflugers Arch. 2019. PMID: 31222490 Review.
Cited by
-
Regulation of ADAM10 activity through microdomain-dependent intracellular calcium changes.Cell Commun Signal. 2024 Nov 4;22(1):531. doi: 10.1186/s12964-024-01891-5. Cell Commun Signal. 2024. PMID: 39497138 Free PMC article.
-
Englerin A Agonizes the TRPC4/C5 Cation Channels to Inhibit Tumor Cell Line Proliferation.PLoS One. 2015 Jun 22;10(6):e0127498. doi: 10.1371/journal.pone.0127498. eCollection 2015. PLoS One. 2015. PMID: 26098886 Free PMC article.
-
Direct modulation of TRPC ion channels by Gα proteins.Front Physiol. 2024 Feb 7;15:1362987. doi: 10.3389/fphys.2024.1362987. eCollection 2024. Front Physiol. 2024. PMID: 38384797 Free PMC article. Review.
-
Unique responses of the fixed stoichiometric TRPC1-TRPC5 concatemer to G proteins.Front Physiol. 2024 Sep 27;15:1392980. doi: 10.3389/fphys.2024.1392980. eCollection 2024. Front Physiol. 2024. PMID: 39397856 Free PMC article.
-
The Role of TRPC1 in Modulating Cancer Progression.Cells. 2020 Feb 7;9(2):388. doi: 10.3390/cells9020388. Cells. 2020. PMID: 32046188 Free PMC article. Review.
References
-
- Koehn FE, Carter GT. Nat Rev Drug Discovery. 2005;4:206–220. - PubMed
- Wilson RM, Danishefsky SJ. J Org Chem. 2006;71:8329–8351. - PubMed
- Kaiser M, Wetzel S, Kumar K, Waldmann H. Cell Mol Life Sci CMLS. 2008;65:1186–1201. - PMC - PubMed
- Bon RS, Waldmann H. Acc Chem Res. 2010;43:1103–1114. - PubMed
- Wetzel S, Bon RS, Kumar K, Waldmann H. Angew Chem. 2011;123:10900–11018. - PubMed
- Newman DJ, Cragg GM. J Nat Prod. 2012;75:311–335. - PMC - PubMed
- van Hattum H, Waldmann H. J Am Chem Soc. 2014;136:11853–11859. - PubMed
- Tao L, Zhu F, Qin C, Zhang C, Xu F, Tan CY, Jiang YY, Chen YZ. Nat Biotechnol. 2014;32:979–980. - PubMed
-
- Bioactivity of (-)Englerin A and analogues
- Ratnayake R, Covell D, Ransom TT, Gustafson KR, Beutler JA. Org Lett. 2009;11:57–60. - PMC - PubMed
- Radtke L, Willot M, Sun HY, Ziegler S, Sauerland S, Strohmann C, Frohlich R, Habenberger P, Waldmann H, Christmann M. Angew Chem. 2011;123:4084. Angew. Chem. Int. Ed. 2011, 50, 3998-4002. - PubMed
- Sulzmaier FJ, Li ZW, Nakashige ML, Fash DM, Chain WJ, Ramos JW. PLoS One. 2012;7 - PMC - PubMed
- Sourbier C, Scroggins BT, Ratnayake R, Prince TL, Lee S, Lee MJ, Nagy PL, Lee YH, Trepel JB, Beutler JA, et al. Cancer Cell. 2013;23:228–237. - PMC - PubMed
- Williams RT, Yu AL, Diccianni MB, Theodorakis EA, Batova A. J Exp Clin Cancer Res. 2013;32 For further studies concerning the bioactivity of Englerin A see ref. 5g. - PMC - PubMed
-
- Total syntheses of (-)Englerin A
- Willot M, Radtke L, Konning D, Frohlich R, Gessner VH, Strohmann C, Christmann M. Angew Chem. 2009;121:9269–9272. Angew. Chem. Int. Ed. 2009, 48, 9105-9108. - PubMed
- Molawi K, Delpont N, Echavarren AM. Angew Chem. 2010;122:3595–3597. Angew. Chem. Int. Ed. 2010, 49, 3517-3519. - PubMed
- Nicolaou KC, Kang QA, Ng SY, Chen DYK. J Am Chem Soc. 2010;132:8219–8222. - PubMed
- Xu J, Caro-Diaz EJE, Theodorakis EA. Org Lett. 2010;12:3708–3711. - PMC - PubMed
- Zhou QH, Chen XF, Ma DW. Angew Chem. 2010;122:3591–3594. Angew. Chem. Int. Ed. 2010, 49, 3513-3516.
- Li ZW, Nakashige M, Chain WJ. J Am Chem Soc. 2011;133:6553–6556. - PubMed
- Pouwer RH, Richard JA, Tseng CC, Chen DYK. Chem Asian J. 2012;7:22–35. - PubMed
- Zahel M, Kessberg A, Metz P. Angew Chem. 2013;125:5500–5502. Angew. Chem. Int. Ed. 2013, 52, 5390-5392. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

