PD-1 blockade induces responses by inhibiting adaptive immune resistance
- PMID: 25428505
- PMCID: PMC4246418
- DOI: 10.1038/nature13954
PD-1 blockade induces responses by inhibiting adaptive immune resistance
Abstract
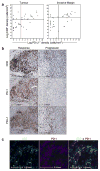
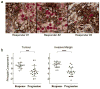
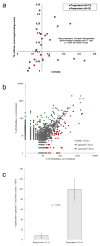
Therapies that target the programmed death-1 (PD-1) receptor have shown unprecedented rates of durable clinical responses in patients with various cancer types. One mechanism by which cancer tissues limit the host immune response is via upregulation of PD-1 ligand (PD-L1) and its ligation to PD-1 on antigen-specific CD8(+) T cells (termed adaptive immune resistance). Here we show that pre-existing CD8(+) T cells distinctly located at the invasive tumour margin are associated with expression of the PD-1/PD-L1 immune inhibitory axis and may predict response to therapy. We analysed samples from 46 patients with metastatic melanoma obtained before and during anti-PD-1 therapy (pembrolizumab) using quantitative immunohistochemistry, quantitative multiplex immunofluorescence, and next-generation sequencing for T-cell antigen receptors (TCRs). In serially sampled tumours, patients responding to treatment showed proliferation of intratumoral CD8(+) T cells that directly correlated with radiographic reduction in tumour size. Pre-treatment samples obtained from responding patients showed higher numbers of CD8-, PD-1- and PD-L1-expressing cells at the invasive tumour margin and inside tumours, with close proximity between PD-1 and PD-L1, and a more clonal TCR repertoire. Using multivariate analysis, we established a predictive model based on CD8 expression at the invasive margin and validated the model in an independent cohort of 15 patients. Our findings indicate that tumour regression after therapeutic PD-1 blockade requires pre-existing CD8(+) T cells that are negatively regulated by PD-1/PD-L1-mediated adaptive immune resistance.
Figures




Similar articles
-
Dynamic Changes in PD-L1 Expression and Immune Infiltrates Early During Treatment Predict Response to PD-1 Blockade in Melanoma.Clin Cancer Res. 2017 Sep 1;23(17):5024-5033. doi: 10.1158/1078-0432.CCR-16-0698. Epub 2017 May 16. Clin Cancer Res. 2017. PMID: 28512174
-
A radiomics approach to assess tumour-infiltrating CD8 cells and response to anti-PD-1 or anti-PD-L1 immunotherapy: an imaging biomarker, retrospective multicohort study.Lancet Oncol. 2018 Sep;19(9):1180-1191. doi: 10.1016/S1470-2045(18)30413-3. Epub 2018 Aug 14. Lancet Oncol. 2018. PMID: 30120041
-
Regulation of PD-L1 expression in a high-grade invasive human oral squamous cell carcinoma microenvironment.Int J Oncol. 2017 Jan;50(1):41-48. doi: 10.3892/ijo.2016.3785. Epub 2016 Dec 2. Int J Oncol. 2017. PMID: 27922697 Free PMC article.
-
Targeting the PD1/PD-L1 axis in melanoma: biological rationale, clinical challenges and opportunities.Crit Rev Oncol Hematol. 2014 Jan;89(1):140-65. doi: 10.1016/j.critrevonc.2013.08.002. Epub 2013 Aug 28. Crit Rev Oncol Hematol. 2014. PMID: 24029602 Review.
-
CTLA-4 and PD-1/PD-L1 blockade: new immunotherapeutic modalities with durable clinical benefit in melanoma patients.Clin Cancer Res. 2013 Oct 1;19(19):5300-9. doi: 10.1158/1078-0432.CCR-13-0143. Clin Cancer Res. 2013. PMID: 24089443 Review.
Cited by
-
Changes in the tumor microenvironment in recurrent head and neck squamous cell carcinoma and its implication on efficacy of immune checkpoint inhibitors.Discov Oncol. 2024 Nov 20;15(1):686. doi: 10.1007/s12672-024-01504-0. Discov Oncol. 2024. PMID: 39567471 Free PMC article.
-
Triggering immunogenic death of cancer cells by nanoparticles overcomes immunotherapy resistance.Cell Oncol (Dordr). 2024 Nov 20. doi: 10.1007/s13402-024-01009-6. Online ahead of print. Cell Oncol (Dordr). 2024. PMID: 39565509 Review.
-
Aberrant expression of MRAS and HEG1 as the biomarkers for osimertinib resistance in LUAD.Discov Oncol. 2024 Nov 19;15(1):678. doi: 10.1007/s12672-024-01552-6. Discov Oncol. 2024. PMID: 39560891 Free PMC article.
-
Stratified analysis identifies HIF-2α as a therapeutic target for highly immune-infiltrated melanomas.bioRxiv [Preprint]. 2024 Oct 30:2024.10.29.620300. doi: 10.1101/2024.10.29.620300. bioRxiv. 2024. PMID: 39554029 Free PMC article. Preprint.
-
Phase I study of a recombinant attenuated oncolytic virus, MEDI5395 (NDV-GM-CSF), administered systemically in combination with durvalumab in patients with advanced solid tumors.J Immunother Cancer. 2024 Nov 17;12(11):e009336. doi: 10.1136/jitc-2024-009336. J Immunother Cancer. 2024. PMID: 39551600 Free PMC article. Clinical Trial.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

