Phase III randomized trial of induction chemotherapy in patients with N2 or N3 locally advanced head and neck cancer
- PMID: 25049329
- PMCID: PMC4876357
- DOI: 10.1200/JCO.2013.54.6309
Phase III randomized trial of induction chemotherapy in patients with N2 or N3 locally advanced head and neck cancer
Abstract
Purpose: Induction chemotherapy (IC) before radiotherapy lowers distant failure (DF) rates in locally advanced squamous cell carcinoma of the head and neck (SCCHN). The goal of this phase III trial was to determine whether IC before chemoradiotherapy (CRT) further improves survival compared with CRT alone in patients with N2 or N3 disease.
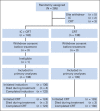
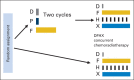
Patients and methods: Treatment-naive patients with nonmetastatic N2 or N3 SCCHN were randomly assigned to CRT alone (CRT arm; docetaxel, fluorouracil, and hydroxyurea plus radiotherapy 0.15 Gy twice per day every other week) versus two 21-day cycles of IC (docetaxel 75 mg/m(2) on day 1, cisplatin 75 mg/m(2) on day 1, and fluorouracil 750 mg/m(2) on days 1 to 5) followed by the same CRT regimen (IC + CRT arm). The primary end point was overall survival (OS). Secondary end points included DF-free survival, failure pattern, and recurrence-free survival (RFS).
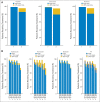
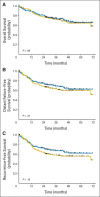
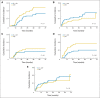
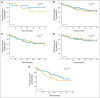
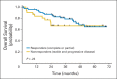
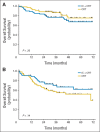
Results: A total of 285 patients were randomly assigned. The most common grade 3 to 4 toxicities during IC were febrile neutropenia (11%) and mucositis (9%); during CRT (both arms combined), they were mucositis (49%), dermatitis (21%), and leukopenia (18%). Serious adverse events were more common in the IC arm (47% v 28%; P = .002). With a minimum follow-up of 30 months, there were no statistically significant differences in OS (hazard ratio, 0.91; 95% CI, 0.59 to 1.41), RFS, or DF-free survival.
Conclusion: IC did not translate into improved OS compared with CRT alone. However, the study was underpowered because it did not meet the planned accrual target, and OS was higher than predicted in both arms. IC cannot be recommended routinely in patients with N2 or N3 locally advanced SCCHN.
© 2014 by American Society of Clinical Oncology.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
Comment in
-
The never-ending story: finding a role for neoadjuvant chemotherapy in the management of head and neck cancer.J Clin Oncol. 2014 Sep 1;32(25):2685-6. doi: 10.1200/JCO.2014.56.7685. Epub 2014 Jul 21. J Clin Oncol. 2014. PMID: 25049324 No abstract available.
-
Reply to s. Chakraborty et al.J Clin Oncol. 2015 Mar 10;33(8):968. doi: 10.1200/JCO.2014.59.6189. Epub 2015 Feb 9. J Clin Oncol. 2015. PMID: 25667282 No abstract available.
-
Does DeCIDE give a decisive answer against induction chemotherapy in head and neck cancers?J Clin Oncol. 2015 Mar 10;33(8):967. doi: 10.1200/JCO.2014.58.3914. Epub 2015 Feb 9. J Clin Oncol. 2015. PMID: 25667296 No abstract available.
Similar articles
-
Induction TPF chemotherapy followed by CRT with fractionated administration of cisplatin in patients with unresectable locally advanced head and neck cancer.Int J Clin Oncol. 2019 Jul;24(7):789-797. doi: 10.1007/s10147-019-01418-w. Epub 2019 Feb 22. Int J Clin Oncol. 2019. PMID: 30796560 Clinical Trial.
-
Induction chemotherapy followed by concurrent chemoradiation in advanced squamous cell carcinoma of the head and neck: final results from a phase II study with docetaxel, cisplatin and 5-fluorouracil with a four-year follow-up.Oral Oncol. 2006 Aug;42(7):675-84. doi: 10.1016/j.oraloncology.2005.12.006. Epub 2006 May 30. Oral Oncol. 2006. PMID: 16731029 Clinical Trial.
-
Induction chemotherapy with docetaxel, cisplatin and 5-fluorouracil followed by radiotherapy with cetuximab for locally advanced squamous cell carcinoma of the head and neck.Eur J Cancer. 2013 Jan;49(2):352-9. doi: 10.1016/j.ejca.2012.08.004. Epub 2012 Sep 14. Eur J Cancer. 2013. PMID: 22981499 Clinical Trial.
-
The Effect of Induction Chemotherapy Using Docetaxel, Cisplatin, and Fluorouracil on Survival in Locally Advanced Head and Neck Squamous Cell Carcinoma: A Meta-Analysis.Cancer Res Treat. 2016 Jul;48(3):907-16. doi: 10.4143/crt.2015.359. Epub 2015 Nov 17. Cancer Res Treat. 2016. PMID: 26582394 Free PMC article. Review.
-
Induction Chemotherapy With 5-Fluorouracil, Cisplatin, and Cetuximab in Advanced Head and Neck Squamous Cell Carcinoma.In Vivo. 2023 May-Jun;37(3):1275-1280. doi: 10.21873/invivo.13205. In Vivo. 2023. PMID: 37103108 Free PMC article. Review.
Cited by
-
[Study results of primary therapy for head and neck tumors : Highlights of the 2016 ASCO Annual Meeting].HNO. 2016 Oct;64(10):717-22. doi: 10.1007/s00106-016-0243-6. HNO. 2016. PMID: 27624903 German.
-
Induction chemotherapy with paclitaxel, carboplatin, and cetuximab (PCE) followed by chemoradiotherapy for unresectable locoregional recurrence after curative surgery in patients with squamous cell carcinoma of the head and neck.Front Oncol. 2024 Jul 1;14:1420860. doi: 10.3389/fonc.2024.1420860. eCollection 2024. Front Oncol. 2024. PMID: 39011480 Free PMC article.
-
Clinical prognostic factors to guide treatment strategy for HPV‑positive oropharyngeal cancer using treatment outcomes of induction chemotherapy: A real‑world experience.Oncol Lett. 2024 Jun 21;28(2):391. doi: 10.3892/ol.2024.14524. eCollection 2024 Aug. Oncol Lett. 2024. PMID: 38966576 Free PMC article.
-
Textural features on 18F-FDG PET/CT and dynamic contrast-enhanced MR imaging for predicting treatment response and survival of patients with hypopharyngeal carcinoma.Medicine (Baltimore). 2019 Aug;98(33):e16608. doi: 10.1097/MD.0000000000016608. Medicine (Baltimore). 2019. PMID: 31415354 Free PMC article.
-
Development and Validation of a Decision Analytical Model for Posttreatment Surveillance for Patients With Oropharyngeal Carcinoma.JAMA Netw Open. 2022 Apr 1;5(4):e227240. doi: 10.1001/jamanetworkopen.2022.7240. JAMA Netw Open. 2022. PMID: 35416988 Free PMC article.
References
-
- Saloura V, Langerman A, Rudra S, et al. Multidisciplinary care of the patient with head and neck cancer. Surg Oncol Clin N Am. 2013;22:179–215. - PubMed
-
- Pignon JP, le Maître A, Maillard E, et al. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): An update on 93 randomised trials and 17,346 patients. Radiother Oncol. 2009;92:4–14. - PubMed
-
- Posner MR, Hershock DM, Blajman CR, et al. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med. 2007;357:1705–1715. - PubMed
-
- Vermorken JB, Remenar E, van Herpen C, et al. Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N Engl J Med. 2007;357:1695–1704. - PubMed
-
- Blanchard P, Bourhis J, Lacas B, et al. Taxane-cisplatin-fluorouracil as induction chemotherapy in locally advanced head and neck cancers: An individual patient data meta-analysis of the meta-analysis of chemotherapy in head and neck cancer group. J Clin Oncol. 2013;31:2854–2860. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

