The genome of the ctenophore Mnemiopsis leidyi and its implications for cell type evolution
- PMID: 24337300
- PMCID: PMC3920664
- DOI: 10.1126/science.1242592
The genome of the ctenophore Mnemiopsis leidyi and its implications for cell type evolution
Abstract
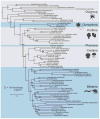
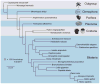
An understanding of ctenophore biology is critical for reconstructing events that occurred early in animal evolution. Toward this goal, we have sequenced, assembled, and annotated the genome of the ctenophore Mnemiopsis leidyi. Our phylogenomic analyses of both amino acid positions and gene content suggest that ctenophores rather than sponges are the sister lineage to all other animals. Mnemiopsis lacks many of the genes found in bilaterian mesodermal cell types, suggesting that these cell types evolved independently. The set of neural genes in Mnemiopsis is similar to that of sponges, indicating that sponges may have lost a nervous system. These results present a newly supported view of early animal evolution that accounts for major losses and/or gains of sophisticated cell types, including nerve and muscle cells.
Figures




Comment in
-
Genetics. My oldest sister is a sea walnut?Science. 2013 Dec 13;342(6164):1327-9. doi: 10.1126/science.1248424. Science. 2013. PMID: 24337283 No abstract available.
-
Evolution: ctenophore genomes and the origin of neurons.Curr Biol. 2014 Aug 18;24(16):R757-61. doi: 10.1016/j.cub.2014.06.057. Curr Biol. 2014. PMID: 25137591
Similar articles
-
Genomic organization, evolution, and expression of photoprotein and opsin genes in Mnemiopsis leidyi: a new view of ctenophore photocytes.BMC Biol. 2012 Dec 21;10:107. doi: 10.1186/1741-7007-10-107. BMC Biol. 2012. PMID: 23259493 Free PMC article.
-
Convergent evolution of neural systems in ctenophores.J Exp Biol. 2015 Feb 15;218(Pt 4):598-611. doi: 10.1242/jeb.110692. J Exp Biol. 2015. PMID: 25696823 Free PMC article. Review.
-
Brief History of Ctenophora.Methods Mol Biol. 2024;2757:1-26. doi: 10.1007/978-1-0716-3642-8_1. Methods Mol Biol. 2024. PMID: 38668961 Review.
-
Studying Ctenophora WBR Using Mnemiopsis leidyi.Methods Mol Biol. 2022;2450:95-119. doi: 10.1007/978-1-0716-2172-1_5. Methods Mol Biol. 2022. PMID: 35359304 Free PMC article.
-
Evolution of the TGF-β signaling pathway and its potential role in the ctenophore, Mnemiopsis leidyi.PLoS One. 2011;6(9):e24152. doi: 10.1371/journal.pone.0024152. Epub 2011 Sep 8. PLoS One. 2011. PMID: 21931657 Free PMC article.
Cited by
-
The premetazoan ancestry of the synaptic toolkit and appearance of first neurons.Essays Biochem. 2022 Dec 8;66(6):781-795. doi: 10.1042/EBC20220042. Essays Biochem. 2022. PMID: 36205407 Free PMC article.
-
Occurrence of Isopenicillin-N-Synthase Homologs in Bioluminescent Ctenophores and Implications for Coelenterazine Biosynthesis.PLoS One. 2015 Jun 30;10(6):e0128742. doi: 10.1371/journal.pone.0128742. eCollection 2015. PLoS One. 2015. PMID: 26125183 Free PMC article.
-
Gene Expression Data from the Moon Jelly, Aurelia, Provide Insights into the Evolution of the Combinatorial Code Controlling Animal Sense Organ Development.PLoS One. 2015 Jul 30;10(7):e0132544. doi: 10.1371/journal.pone.0132544. eCollection 2015. PLoS One. 2015. PMID: 26225420 Free PMC article.
-
Cancer across the tree of life: cooperation and cheating in multicellularity.Philos Trans R Soc Lond B Biol Sci. 2015 Jul 19;370(1673):20140219. doi: 10.1098/rstb.2014.0219. Philos Trans R Soc Lond B Biol Sci. 2015. PMID: 26056363 Free PMC article. Review.
-
Metabolic and chaperone gene loss marks the origin of animals: evidence for Hsp104 and Hsp78 chaperones sharing mitochondrial enzymes as clients.PLoS One. 2015 Feb 24;10(2):e0117192. doi: 10.1371/journal.pone.0117192. eCollection 2015. PLoS One. 2015. PMID: 25710177 Free PMC article.
References
-
- Chapman JA, Kirkness EF, Simakov O, Hampson SE, Mitros T, Weinmaier T, Rattei T, Balasubramanian PG, Borman J, Busam D, Disbennett K, Pfannkoch C, Sumin N, Sutton GG, Viswanathan LD, Walenz B, Goodstein DM, Hellsten U, Kawashima T, Prochnik SE, Putnam NH, Shu S, Blumberg B, Dana CE, Gee L, Kibler DF, Law L, Lindgens D, Martinez DE, Peng J, Wigge PA, Bertulat B, Guder C, Nakamura Y, Ozbek S, Watanabe H, Khalturin K, Hemmrich G, Franke A, Augustin R, Fraune S, Hayakawa E, Hayakawa S, Hirose M, Hwang JS, Ikeo K, Nishimiya-Fujisawa C, Ogura A, Takahashi T, Steinmetz PR, Zhang X, Aufschnaiter R, Eder MK, Gorny AK, Salvenmoser W, Heimberg AM, Wheeler BM, Peterson KJ, Bottger A, Tischler P, Wolf A, Gojobori T, Remington KA, Strausberg RL, Venter JC, Technau U, Hobmayer B, Bosch TC, Holstein TW, Fujisawa T, Bode HR, David CN, Rokhsar DS, Steele RE. The dynamic genome of Hydra. Nature. 2010;464:592–596. doi: 10.1038/nature08830. - DOI - PMC - PubMed
-
- Putnam NH, Srivastava M, Hellsten U, Dirks B, Chapman J, Salamov A, Terry A, Shapiro H, Lindquist E, Kapitonov VV, Jurka J, Genikhovich G, Grigoriev IV, Lucas SM, Steele RE, Finnerty JR, Technau U, Martindale MQ, Rokhsar DS. Sea anemone genome reveals ancestral eumetazoan gene repertoire and genomic organization. Science. 2007;317:86–94. - PubMed
-
- Srivastava M, Begovic E, Chapman J, Putnam NH, Hellsten U, Kawashima T, Kuo A, Mitros T, Salamov A, Carpenter ML, Signorovitch AY, Moreno MA, Kamm K, Grimwood J, Schmutz J, Shapiro H, Grigoriev IV, Buss LW, Schierwater B, Dellaporta SL, Rokhsar DS. The Trichoplax genome and the nature of placozoans. Nature. 2008;454:955–960. - PubMed
-
- Srivastava M, Simakov O, Chapman J, Fahey B, Gauthier ME, Mitros T, Richards GS, Conaco C, Dacre M, Hellsten U, Larroux C, Putnam NH, Stanke M, Adamska M, Darling A, Degnan SM, Oakley TH, Plachetzki DC, Zhai Y, Adamski M, Calcino A, Cummins SF, Goodstein DM, Harris C, Jackson DJ, Leys SP, Shu S, Woodcroft BJ, Vervoort M, Kosik KS, Manning G, Degnan BM, Rokhsar DS. The Amphimedon queenslandica genome and the evolution of animal complexity. Nature. 2010;466:720–726. - PMC - PubMed
-
- King N, Westbrook MJ, Young SL, Kuo A, Abedin M, Chapman J, Fairclough S, Hellsten U, Isogai Y, Letunic I, Marr M, Pincus D, Putnam N, Rokas A, Wright KJ, Zuzow R, Dirks W, Good M, Goodstein D, Lemons D, Li W, Lyons JB, Morris A, Nichols S, Richter DJ, Salamov A, Sequencing JG, Bork P, Lim WA, Manning G, Miller WT, McGinnis W, Shapiro H, Tjian R, Grigoriev IV, Rokhsar D. The genome of the choanoflagellate Monosiga brevicollis and the origin of metazoans. Nature. 2008;451:783–788. - PMC - PubMed
Publication types
MeSH terms
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

