A pilot study assessing the safety and latency-reversing activity of disulfiram in HIV-1-infected adults on antiretroviral therapy
- PMID: 24336828
- PMCID: PMC3935499
- DOI: 10.1093/cid/cit813
A pilot study assessing the safety and latency-reversing activity of disulfiram in HIV-1-infected adults on antiretroviral therapy
Abstract
Background: Transcriptionally silent human immunodeficiency virus type 1 (HIV-1) DNA persists in resting memory CD4(+) T cells despite antiretroviral therapy. In a primary cell model, the antialcoholism drug disulfiram has been shown to induce HIV-1 transcription in latently infected resting memory CD4(+) T cells at concentrations achieved in vivo.
Methods: We conducted a single-arm pilot study to evaluate whether 500 mg of disulfiram administered daily for 14 days to HIV-1-infected individuals on stable suppressive antiretroviral therapy would result in reversal of HIV-1 latency with a concomitant transient increase in residual viremia or depletion of the latent reservoir in resting memory CD4(+) T cells.
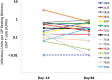
Results: Disulfiram was safe and well tolerated. There was a high level of subject-to-subject variability in plasma disulfiram levels. The latent reservoir did not change significantly (1.16-fold change; 95% confidence interval [CI], .70- to 1.92-fold; P = .56). During disulfiram administration, residual viremia did not change significantly compared to baseline (1.53-fold; 95% CI, .88- to 2.69-fold; P = .13), although residual viremia was estimated to increase by 1.88-fold compared to baseline during the postdosing period (95% CI, 1.03- to 3.43-fold; P = .04). In a post hoc analysis, a rapid and transient increase in viremia was noted in a subset of individuals (n = 6) with immediate postdose sampling (HIV-1 RNA increase, 2.96-fold; 95% CI, 1.29- to 6.81-fold; P = .01).
Conclusions: Administration of disulfiram to patients on antiretroviral therapy does not reduce the size of the latent reservoir. A possible dose-related effect on residual viremia supports future studies assessing the impact of higher doses on HIV-1 production. Disulfiram affects relevant signaling pathways and can be safely administered, supporting future studies of this drug.
Keywords: HIV-1 latent reservoir; disulfiram; latency-reversing agents.
Figures

Similar articles
-
Short-term administration of disulfiram for reversal of latent HIV infection: a phase 2 dose-escalation study.Lancet HIV. 2015 Dec;2(12):e520-9. doi: 10.1016/S2352-3018(15)00226-X. Epub 2015 Nov 17. Lancet HIV. 2015. PMID: 26614966 Free PMC article. Clinical Trial.
-
Maraviroc Is Associated with Latent HIV-1 Reactivation through NF-κB Activation in Resting CD4+ T Cells from HIV-Infected Individuals on Suppressive Antiretroviral Therapy.J Virol. 2018 Apr 13;92(9):e01931-17. doi: 10.1128/JVI.01931-17. Print 2018 May 1. J Virol. 2018. PMID: 29444937 Free PMC article.
-
The effect of intensification with raltegravir on the HIV-1 reservoir of latently infected memory CD4 T cells in suppressed patients.AIDS. 2012 Sep 24;26(15):1885-94. doi: 10.1097/QAD.0b013e3283584521. AIDS. 2012. PMID: 22992577 Clinical Trial.
-
Therapeutic Approaches to Eradicate Latent HIV-1 in Resting CD4+ T Cells.Curr Top Med Chem. 2016;16(10):1191-7. doi: 10.2174/1568026615666150901114138. Curr Top Med Chem. 2016. PMID: 26324046 Review.
-
HIV-1 Reservoirs During Suppressive Therapy.Trends Microbiol. 2016 May;24(5):345-355. doi: 10.1016/j.tim.2016.01.006. Epub 2016 Feb 12. Trends Microbiol. 2016. PMID: 26875617 Free PMC article. Review.
Cited by
-
Getting the "Kill" into "Shock and Kill": Strategies to Eliminate Latent HIV.Cell Host Microbe. 2018 Jan 10;23(1):14-26. doi: 10.1016/j.chom.2017.12.004. Cell Host Microbe. 2018. PMID: 29324227 Free PMC article. Review.
-
Eradicating HIV-1 infection: seeking to clear a persistent pathogen.Nat Rev Microbiol. 2014 Nov;12(11):750-64. doi: 10.1038/nrmicro3352. Nat Rev Microbiol. 2014. PMID: 25402363 Free PMC article. Review.
-
Multiple Origins of Virus Persistence during Natural Control of HIV Infection.Cell. 2016 Aug 11;166(4):1004-1015. doi: 10.1016/j.cell.2016.06.039. Epub 2016 Jul 21. Cell. 2016. PMID: 27453467 Free PMC article.
-
Toward development of epigenetic drugs for central nervous system disorders: Modulating neuroplasticity via H3K4 methylation.Psychiatry Clin Neurosci. 2016 Dec;70(12):536-550. doi: 10.1111/pcn.12426. Epub 2016 Sep 7. Psychiatry Clin Neurosci. 2016. PMID: 27485392 Free PMC article. Review.
-
Fimepinostat, a novel dual inhibitor of HDAC and PI3K, effectively reverses HIV-1 latency ex vivo without T cell activation.J Virus Erad. 2019 Sep 18;5(3):133-137. doi: 10.1016/S2055-6640(20)30042-X. J Virus Erad. 2019. PMID: 31700655 Free PMC article.
References
-
- Moore RD, Keruly JC, Gebo KA, Lucas GM. An improvement in virologic response to highly active antiretroviral therapy in clinical practice from 1996 through 2002. J Acquir Immune Defic Syndr. 2005;39:195–8. - PubMed
-
- Perelson AS, Essunger P, Cao Y, et al. Decay characteristics of HIV-1-infected compartments during combination therapy. Nature. 1997;387:188–91. - PubMed
-
- Wong JK, Hezareh M, Gunthard HF, et al. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science. 1997;278:1291–5. - PubMed
-
- Finzi D, Hermankova M, Pierson T, et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997;278:1295–300. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 MH062246/MH/NIMH NIH HHS/United States
- P30 AI094189/AI/NIAID NIH HHS/United States
- U19 AI096109/AI/NIAID NIH HHS/United States
- UL1 TR 000424-06/TR/NCATS NIH HHS/United States
- UL1 RR024131/RR/NCRR NIH HHS/United States
- U19 AI096113/AI/NIAID NIH HHS/United States
- P30 MH62246/MH/NIMH NIH HHS/United States
- AI096113/AI/NIAID NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- K24 AI069994/AI/NIAID NIH HHS/United States
- 1U19AI096109/AI/NIAID NIH HHS/United States
- UL1 TR000424/TR/NCATS NIH HHS/United States
- P30 AI027763/AI/NIAID NIH HHS/United States
- 43222/PHS HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

