Proteasomal degradation of Nck1 but not Nck2 regulates RhoA activation and actin dynamics
- PMID: 24287595
- PMCID: PMC4698162
- DOI: 10.1038/ncomms3863
Proteasomal degradation of Nck1 but not Nck2 regulates RhoA activation and actin dynamics
Abstract
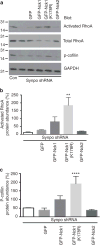
The ubiquitously expressed adapter proteins Nck1/2 interact with a multitude of effector molecules to regulate diverse cellular functions including cytoskeletal dynamics. Here we show that Nck1, but not Nck2, is a substrate of c-Cbl-mediated ubiquitination. We uncover lysine 178 in Nck1 as the evolutionarily conserved ubiquitin acceptor site. We previously reported that synaptopodin, a proline-rich actin-binding protein, induces stress fibres by blocking the Smurf1-mediated ubiquitination of RhoA. We now find that synaptopodin competes with c-Cbl for binding to Nck1, which prevents the ubiquitination of Nck1 by c-Cbl. Gene silencing of c-Cbl restores Nck1 protein abundance and stress fibres in synaptopodin knockdown cells. Similarly, expression of c-Cbl-resistant Nck1(K178R) or Nck2 containing the SH3 domain 2 of Nck1 restores stress fibres in synaptopodin-depleted podocytes through activation of RhoA signalling. These findings reveal proteasomal regulation as a key factor in the distinct and non-redundant effects of Nck on RhoA-mediated actin dynamics.
Conflict of interest statement
Figures


Similar articles
-
Ubiquitylation-dependent downregulation of Nck regulates its functional activity.FEBS Lett. 2014 Nov 3;588(21):3808-15. doi: 10.1016/j.febslet.2014.08.033. Epub 2014 Sep 16. FEBS Lett. 2014. PMID: 25218436
-
Complementary Nck1/2 Signaling in Podocytes Controls α Actinin-4-Mediated Actin Organization, Adhesion, and Basement Membrane Composition.J Am Soc Nephrol. 2022 Aug;33(8):1546-1567. doi: 10.1681/ASN.2021101343. J Am Soc Nephrol. 2022. PMID: 35906089 Free PMC article.
-
Nck1, But Not Nck2, Mediates Disturbed Flow-Induced p21-Activated Kinase Activation and Endothelial Permeability.J Am Heart Assoc. 2020 Jun 2;9(11):e016099. doi: 10.1161/JAHA.120.016099. Epub 2020 May 29. J Am Heart Assoc. 2020. PMID: 32468886 Free PMC article.
-
Adapting to change: resolving the dynamic and dual roles of NCK1 and NCK2.Biochem J. 2024 Oct 16;481(20):1411-1435. doi: 10.1042/BCJ20230232. Biochem J. 2024. PMID: 39392452 Review.
-
Sinner or Saint?: Nck Adaptor Proteins in Vascular Biology.Front Cell Dev Biol. 2021 May 26;9:688388. doi: 10.3389/fcell.2021.688388. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34124074 Free PMC article. Review.
Cited by
-
Mechanism of Bile Acid in Regulating Platelet Function and Thrombotic Diseases.Adv Sci (Weinh). 2024 Aug;11(32):e2401683. doi: 10.1002/advs.202401683. Epub 2024 Jun 23. Adv Sci (Weinh). 2024. PMID: 38922767 Free PMC article.
-
Cholesterol and matrisome pathways dysregulated in astrocytes and microglia.Cell. 2022 Jun 23;185(13):2213-2233.e25. doi: 10.1016/j.cell.2022.05.017. Cell. 2022. PMID: 35750033 Free PMC article.
-
Nck1 promotes the progression of ovarian carcinoma by enhancing the PI3K/AKT/p70S6K signaling.Hum Cell. 2020 Jul;33(3):768-779. doi: 10.1007/s13577-020-00344-8. Epub 2020 Mar 12. Hum Cell. 2020. PMID: 32166565
-
Role of Pirin, an Oxidative Stress Sensor Protein, in Epithelial Carcinogenesis.Biology (Basel). 2021 Feb 4;10(2):116. doi: 10.3390/biology10020116. Biology (Basel). 2021. PMID: 33557375 Free PMC article. Review.
-
Revisiting Non-BRCA1/2 Familial Whole Exome Sequencing Datasets Implicates NCK1 as a Cancer Gene.Front Genet. 2019 Jun 4;10:527. doi: 10.3389/fgene.2019.00527. eCollection 2019. Front Genet. 2019. PMID: 31214250 Free PMC article.
References
-
- Buday L, Wunderlich L, Tamas P. The Nck family of adapter proteins: regulators of actin cytoskeleton. Cell Signal. 2002;14:723–731. - PubMed
-
- Rohatgi R, Nollau P, Ho HY, Kirschner MW, Mayer BJ. Nck and phosphatidylinositol 4,5-bisphosphate synergistically activate actin polymerization through the N-WASP-Arp2/3 pathway. J Biol Chem. 2001;276:26448–26452. - PubMed
-
- Lu W, Katz S, Gupta R, Mayer BJ. Activation of Pak by membrane localization mediated by an SH3 domain from the adaptor protein Nck. Curr Biol. 1997;7:85–94. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

