Identification of retinal ganglion cell neuroprotection conferred by platelet-derived growth factor through analysis of the mesenchymal stem cell secretome
- PMID: 24176979
- PMCID: PMC3914467
- DOI: 10.1093/brain/awt292
Identification of retinal ganglion cell neuroprotection conferred by platelet-derived growth factor through analysis of the mesenchymal stem cell secretome
Abstract
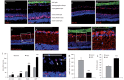
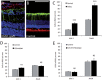
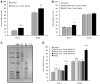
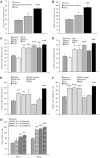
The development of neuroprotective strategies to attenuate retinal ganglion cell death could lead to novel therapies for chronic optic neuropathies such as glaucoma. Intravitreal transplantation of mesenchymal stem cells slows retinal ganglion cell death in models of optic nerve injury, but the mechanism of action remains unclear. Here we characterized the neuroprotective effects of mesenchymal stem cells and mesenchymal stem cell-derived factors in organotypic retinal explant culture and an in vivo model of ocular hypertensive glaucoma. Co-culture of rat and human bone marrow-derived mesenchymal stem cells with retinal explants increased retinal ganglion cell survival, after 7 days ex vivo, by ∼2-fold and was associated with reduced apoptosis and increased nerve fibre layer and inner plexiform layer thicknesses. These effects were not demonstrated by co-culture with human or mouse fibroblasts. Conditioned media from mesenchymal stem cells conferred neuroprotection, suggesting that the neuroprotection is mediated, at least partly, by secreted factors. We compared the concentrations of 29 factors in human mesenchymal stem cell and fibroblast conditioned media, and identified 11 enriched in the mesenchymal stem cell secretome. Treatment of retinal explants with a cocktail of these factors conferred retinal ganglion cell neuroprotection, with factors from the platelet-derived growth factor family being the most potent. Blockade of platelet-derived growth factor signalling with neutralizing antibody or with small molecule inhibitors of platelet-derived growth factor receptor kinase or downstream phosphatidylinositol 3 kinase eliminated retinal ganglion cell neuroprotection conferred by mesenchymal stem cell co-culture. Intravitreal injection of platelet-derived growth factor -AA or -AB led to profound optic nerve neuroprotection in vivo following experimental induction of elevated intraocular pressure. These data demonstrate that mesenchymal stem cells secrete a number of neuroprotective proteins and suggest that platelet-derived growth factor secretion in particular may play an important role in mesenchymal stem cell-mediated retinal ganglion cell neuroprotection. Furthermore, platelet-derived growth factor may represent an independent target for achieving retinal ganglion cell neuroprotection.
Keywords: glaucoma; mesenchymal stem cell; neuroprotection; platelet derived growth factor; retinal ganglion cell.
Figures

Comment in
-
Reply: Platelet-derived growth factor-BB may be involved in mesenchymal stem cell secretome-induced neuroprotection of retinal ganglion cells.Brain. 2014 May;137(Pt 5):e277. doi: 10.1093/brain/awu011. Epub 2014 Feb 14. Brain. 2014. PMID: 24531624 No abstract available.
-
Platelet-derived growth factor-BB is involved in mesenchymal stem cell secretome-induced neuroprotection of retinal ganglion cells.Brain. 2014 May;137(Pt 5):e276. doi: 10.1093/brain/awu017. Epub 2014 Feb 20. Brain. 2014. PMID: 24561518 No abstract available.
Similar articles
-
Neuroprotective Effects of Human Mesenchymal Stem Cells and Platelet-Derived Growth Factor on Human Retinal Ganglion Cells.Stem Cells. 2018 Jan;36(1):65-78. doi: 10.1002/stem.2722. Epub 2017 Oct 31. Stem Cells. 2018. PMID: 29044808 Free PMC article.
-
Paracrine-mediated neuroprotection and neuritogenesis of axotomised retinal ganglion cells by human dental pulp stem cells: comparison with human bone marrow and adipose-derived mesenchymal stem cells.PLoS One. 2014 Oct 7;9(10):e109305. doi: 10.1371/journal.pone.0109305. eCollection 2014. PLoS One. 2014. PMID: 25290916 Free PMC article.
-
Use of an adult rat retinal explant model for screening of potential retinal ganglion cell neuroprotective therapies.Invest Ophthalmol Vis Sci. 2011 May 17;52(6):3309-20. doi: 10.1167/iovs.10-6873. Invest Ophthalmol Vis Sci. 2011. PMID: 21345987 Free PMC article.
-
Molecular and cell-based approaches for neuroprotection in glaucoma.Optom Vis Sci. 2008 Jun;85(6):417-24. doi: 10.1097/OPX.0b013e31817841f7. Optom Vis Sci. 2008. PMID: 18521011 Review.
-
Protecting retinal ganglion cells.Eye (Lond). 2017 Feb;31(2):218-224. doi: 10.1038/eye.2016.299. Epub 2017 Jan 13. Eye (Lond). 2017. PMID: 28085136 Free PMC article. Review.
Cited by
-
Advances in Neuroprotection in Glaucoma: Pharmacological Strategies and Emerging Technologies.Pharmaceuticals (Basel). 2024 Sep 25;17(10):1261. doi: 10.3390/ph17101261. Pharmaceuticals (Basel). 2024. PMID: 39458902 Free PMC article. Review.
-
Glaucoma: Current and New Therapeutic Approaches.Biomedicines. 2024 Sep 3;12(9):2000. doi: 10.3390/biomedicines12092000. Biomedicines. 2024. PMID: 39335514 Free PMC article. Review.
-
Methods to Investigate the Secretome of Senescent Cells.Methods Protoc. 2024 Jul 2;7(4):52. doi: 10.3390/mps7040052. Methods Protoc. 2024. PMID: 39051266 Free PMC article. Review.
-
Mesenchymal stem cells for repairing glaucomatous optic nerve.Int J Ophthalmol. 2024 Apr 18;17(4):748-760. doi: 10.18240/ijo.2024.04.20. eCollection 2024. Int J Ophthalmol. 2024. PMID: 38638254 Free PMC article. Review.
-
Early detection of optic nerve head changes using optical coherence tomography after using mesenchymal stromal cells as intravitreal therapy in rabbit models of ocular hypertension.Vet World. 2024 Feb;17(2):500-508. doi: 10.14202/vetworld.2024.500-508. Epub 2024 Feb 29. Vet World. 2024. PMID: 38595669 Free PMC article.
References
-
- Almasieh M, Wilson AM, Morquette B, Cueva Vargas JL, Di Polo A. The molecular basis of retinal ganglion cell death in glaucoma. Prog Retin Eye Res. 2012;31:152–81. - PubMed
-
- Bampton ET, Ma CH, Tolkovsky AM, Taylor JS. Osteonectin is a Schwann cell-secreted factor that promotes retinal ganglion cell survival and process outgrowth. Eur J Neurosci. 2005;21:2611–23. - PubMed
-
- Biswas SK, Zhao Y, Nagalingam A, Gardner TW, Sandirasegarane L. PDGF- and insulin/IGF-1-specific distinct modes of class IA PI 3-kinase activation in normal rat retinas and RGC-5 retinal ganglion cells. Invest Ophthalmol Vis Sci. 2008;49:3687–98. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

