Advances in the understanding of mammalian copper transporters
- PMID: 22332042
- PMCID: PMC3065767
- DOI: 10.3945/an.110.000273
Advances in the understanding of mammalian copper transporters
Abstract
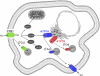
Copper (Cu) is an essential micronutrient. Its ability to exist in 2 oxidation states (Cu(1+) and Cu(2+)) allows it to function as an enzymatic cofactor in hydrolytic, electron transfer, and oxygen utilization reactions. Cu transporters CTR1, ATP7A, and ATP7B play key roles in ensuring that adequate Cu is available for Cu-requiring processes and the prevention of excess Cu accumulation within cells. Two diseases of Cu metabolism, Menkes disease and Wilson disease, which are caused by mutations in ATP7A and ATP7B, respectively, exemplify the critical importance of regulating Cu balance in humans. Herein, we review recent studies of the biochemical and cell biological characteristics of CTR1, ATP7A, and ATP7B, as well as emerging roles for Cu in new areas of physiology.
Conflict of interest statement
Author disclosures: Y. Wang, V. Hodgkinson, S. Zhu, G. A. Weisman, and M. J. Petris, no conflicts of interest.
Figures
Similar articles
-
[Structure and function of ATP7A and ATP7B proteins--Cu-transporting ATPases].Postepy Biochem. 2010;56(3):317-27. Postepy Biochem. 2010. PMID: 21117320 Review. Polish.
-
Copper transporting P-type ATPases and human disease.J Bioenerg Biomembr. 2002 Oct;34(5):333-8. doi: 10.1023/a:1021293818125. J Bioenerg Biomembr. 2002. PMID: 12539960 Review.
-
[From gene to disease: copper-transporting P ATPases alteration].Pathol Biol (Paris). 2009 May;57(3):272-9. doi: 10.1016/j.patbio.2008.09.004. Epub 2008 Nov 28. Pathol Biol (Paris). 2009. PMID: 19046832 French.
-
Clusterin (apolipoprotein J), a molecular chaperone that facilitates degradation of the copper-ATPases ATP7A and ATP7B.J Biol Chem. 2011 Mar 25;286(12):10073-83. doi: 10.1074/jbc.M110.190546. Epub 2011 Jan 17. J Biol Chem. 2011. PMID: 21242307 Free PMC article.
-
Clusterin and COMMD1 independently regulate degradation of the mammalian copper ATPases ATP7A and ATP7B.J Biol Chem. 2012 Jan 20;287(4):2485-99. doi: 10.1074/jbc.M111.302216. Epub 2011 Nov 30. J Biol Chem. 2012. PMID: 22130675 Free PMC article.
Cited by
-
Activity and Trafficking of Copper-Transporting ATPases in Tumor Development and Defense against Platinum-Based Drugs.Cells. 2019 Sep 13;8(9):1080. doi: 10.3390/cells8091080. Cells. 2019. PMID: 31540259 Free PMC article. Review.
-
Ligand-Doped Copper Oxo-hydroxide Nanoparticles are Effective Antimicrobials.Nanoscale Res Lett. 2018 Apr 19;13(1):111. doi: 10.1186/s11671-018-2520-7. Nanoscale Res Lett. 2018. PMID: 29675656 Free PMC article.
-
Eat Prey, Live: Dictyostelium discoideum As a Model for Cell-Autonomous Defenses.Front Immunol. 2018 Jan 4;8:1906. doi: 10.3389/fimmu.2017.01906. eCollection 2017. Front Immunol. 2018. PMID: 29354124 Free PMC article. Review.
-
Bacterial Proteasomes: Mechanistic and Functional Insights.Microbiol Mol Biol Rev. 2016 Dec 14;81(1):e00036-16. doi: 10.1128/MMBR.00036-16. Print 2017 Mar. Microbiol Mol Biol Rev. 2016. PMID: 27974513 Free PMC article. Review.
-
Cellular sensing and transport of metal ions: implications in micronutrient homeostasis.J Nutr Biochem. 2015 Nov;26(11):1103-15. doi: 10.1016/j.jnutbio.2015.08.002. Epub 2015 Aug 7. J Nutr Biochem. 2015. PMID: 26342943 Free PMC article. Review.
References
-
- Andreini C, Banci L, Bertini I, Rosato A. Occurrence of copper proteins through the three domains of life: a bioinformatic approach. J Proteome Res. 2008;7:209–16 - PubMed
-
- Puig S, Lee J, Lau M, Thiele DJ. Biochemical and genetic analyses of yeast and human high affinity copper transporters suggest a conserved mechanism for copper uptake. J Biol Chem. 2002;277:26021–30 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

