Clusterin and COMMD1 independently regulate degradation of the mammalian copper ATPases ATP7A and ATP7B
- PMID: 22130675
- PMCID: PMC3268409
- DOI: 10.1074/jbc.M111.302216
Clusterin and COMMD1 independently regulate degradation of the mammalian copper ATPases ATP7A and ATP7B
Abstract
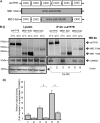
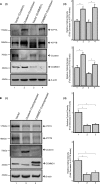
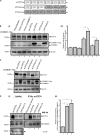
ATP7A and ATP7B are copper-transporting P(1B)-type ATPases (Cu-ATPases) that are critical for regulating intracellular copper homeostasis. Mutations in the genes encoding ATP7A and ATP7B lead to copper deficiency and copper toxicity disorders, Menkes and Wilson diseases, respectively. Clusterin and COMMD1 were previously identified as interacting partners of these Cu-ATPases. In this study, we confirmed that clusterin and COMMD1 interact to down-regulate both ATP7A and ATP7B. Overexpression and knockdown of clusterin/COMMD1 decreased and increased, respectively, endogenous levels of ATP7A and ATP7B, consistent with a role in facilitating Cu-ATPase degradation. We demonstrate that whereas the clusterin/ATP7B interaction was enhanced by oxidative stress or mutation of ATP7B, the COMMD1/ATP7B interaction did not change under oxidative stress conditions, and only increased with ATP7B mutations that led to its misfolding. Clusterin and COMMD1 facilitated the degradation of ATP7B containing the same Wilson disease-causing C-terminal mutations via different degradation pathways, clusterin via the lysosomal pathway and COMMD1 via the proteasomal pathway. Furthermore, endogenous ATP7B existed in a complex with clusterin and COMMD1, but these interactions were neither competitive nor cooperative and occurred independently of each other. Together these data indicate that clusterin and COMMD1 represent alternative and independent systems regulating Cu-ATPase quality control, and consequently contributing to the maintenance of copper homeostasis.
Figures




Similar articles
-
Clusterin (apolipoprotein J), a molecular chaperone that facilitates degradation of the copper-ATPases ATP7A and ATP7B.J Biol Chem. 2011 Mar 25;286(12):10073-83. doi: 10.1074/jbc.M110.190546. Epub 2011 Jan 17. J Biol Chem. 2011. PMID: 21242307 Free PMC article.
-
The copper-transporting capacity of ATP7A mutants associated with Menkes disease is ameliorated by COMMD1 as a result of improved protein expression.Cell Mol Life Sci. 2012 Jan;69(1):149-63. doi: 10.1007/s00018-011-0743-1. Epub 2011 Jun 11. Cell Mol Life Sci. 2012. PMID: 21667063 Free PMC article.
-
Distinct Wilson's disease mutations in ATP7B are associated with enhanced binding to COMMD1 and reduced stability of ATP7B.Gastroenterology. 2007 Oct;133(4):1316-26. doi: 10.1053/j.gastro.2007.07.020. Epub 2007 Jul 25. Gastroenterology. 2007. PMID: 17919502 Free PMC article.
-
Copper transporting P-type ATPases and human disease.J Bioenerg Biomembr. 2002 Oct;34(5):333-8. doi: 10.1023/a:1021293818125. J Bioenerg Biomembr. 2002. PMID: 12539960 Review.
-
[Structure and function of ATP7A and ATP7B proteins--Cu-transporting ATPases].Postepy Biochem. 2010;56(3):317-27. Postepy Biochem. 2010. PMID: 21117320 Review. Polish.
Cited by
-
The Antitumor Peptide CIGB-552 Increases COMMD1 and Inhibits Growth of Human Lung Cancer Cells.J Amino Acids. 2013;2013:251398. doi: 10.1155/2013/251398. Epub 2013 Jan 16. J Amino Acids. 2013. PMID: 23401744 Free PMC article.
-
Triptolide-induced cuproptosis is a novel antitumor strategy for the treatment of cervical cancer.Cell Mol Biol Lett. 2024 Aug 28;29(1):113. doi: 10.1186/s11658-024-00623-4. Cell Mol Biol Lett. 2024. PMID: 39198750 Free PMC article.
-
Copper Homeostasis in Mammals, with Emphasis on Secretion and Excretion. A Review.Int J Mol Sci. 2020 Jul 13;21(14):4932. doi: 10.3390/ijms21144932. Int J Mol Sci. 2020. PMID: 32668621 Free PMC article. Review.
-
Stress-responsive regulation of extracellular proteostasis.J Cell Biol. 2022 Apr 4;221(4):e202112104. doi: 10.1083/jcb.202112104. Epub 2022 Feb 22. J Cell Biol. 2022. PMID: 35191945 Free PMC article. Review.
-
Role of the P-Type ATPases, ATP7A and ATP7B in brain copper homeostasis.Front Aging Neurosci. 2013 Aug 23;5:44. doi: 10.3389/fnagi.2013.00044. eCollection 2013. Front Aging Neurosci. 2013. PMID: 23986700 Free PMC article.
References
-
- Lutsenko S., Petrukhin K., Cooper M. J., Gilliam C. T., Kaplan J. H. (1997) N-terminal domains of human copper-transporting adenosine triphosphatases (the Wilson and Menkes disease proteins) bind copper selectively in vivo and in vitro with stoichiometry of one copper per metal-binding repeat. J. Biol. Chem. 272, 18939–18944 - PubMed
-
- Lutsenko S., Barnes N. L., Bartee M. Y., Dmitriev O. Y. (2007) Function and regulation of human copper-transporting ATPases. Physiol. Rev. 87, 1011–1046 - PubMed
-
- Monty J. F., Llanos R. M., Mercer J. F., Kramer D. R. (2005) Copper exposure induces trafficking of the Menkes protein in intestinal epithelium of ATP7A transgenic mice. J. Nutr. 135, 2762–2766 - PubMed
-
- Nyasae L., Bustos R., Braiterman L., Eipper B., Hubbard A. (2007) Dynamics of endogenous ATP7A (Menkes protein) in intestinal epithelial cells. Copper-dependent redistribution between two intracellular sites. Am. J. Physiol. Gastrointest. Liver Physiol. 292, G1181–1194 - PubMed
-
- Roelofsen H., Wolters H., Van Luyn M. J., Miura N., Kuipers F., Vonk R. J. (2000) Copper-induced apical trafficking of ATP7B in polarized hepatoma cells provides a mechanism for biliary copper excretion. Gastroenterology 119, 782–793 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

