Individual caspase-10 isoforms play distinct and opposing roles in the initiation of death receptor-mediated tumour cell apoptosis
- PMID: 21368896
- PMCID: PMC3101821
- DOI: 10.1038/cddis.2011.8
Individual caspase-10 isoforms play distinct and opposing roles in the initiation of death receptor-mediated tumour cell apoptosis
Abstract
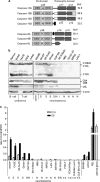
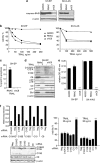
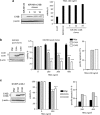
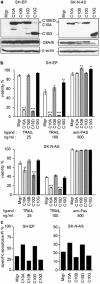
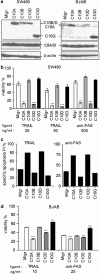
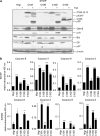
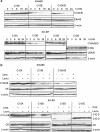
The cysteine protease caspase-8 is an essential executioner of the death receptor (DR) apoptotic pathway. The physiological function of its homologue caspase-10 remains poorly understood, and the ability of caspase-10 to substitute for caspase-8 in the DR apoptotic pathway is still controversial. Here, we analysed the particular contribution of caspase-10 isoforms to DR-mediated apoptosis in neuroblastoma (NB) cells characterised by their resistance to DR signalling. Silencing of caspase-8 in tumour necrosis factor-related apoptosis-inducing ligand (TRAIL)-sensitive NB cells resulted in complete resistance to TRAIL, which could be reverted by overexpression of caspase-10A or -10D. Overexpression experiments in various caspase-8-expressing tumour cells also demonstrated that caspase-10A and -10D isoforms strongly increased TRAIL and FasL sensitivity, whereas caspase-10B or -10G had no effect or were weakly anti-apoptotic. Further investigations revealed that the unique C-terminal end of caspase-10B was responsible for its degradation by the ubiquitin-proteasome pathway and for its lack of pro-apoptotic activity compared with caspase-10A and -10D. These data highlight in several tumour cell types, a differential pro- or anti-apoptotic role for the distinct caspase-10 isoforms in DR signalling, which may be relevant for fine tuning of apoptosis initiation.
Figures

Similar articles
-
Cellular FLICE-inhibitory protein (cFLIP) isoforms block CD95- and TRAIL death receptor-induced gene induction irrespective of processing of caspase-8 or cFLIP in the death-inducing signaling complex.J Biol Chem. 2011 May 13;286(19):16631-46. doi: 10.1074/jbc.M110.148585. Epub 2011 Mar 22. J Biol Chem. 2011. PMID: 21454681 Free PMC article.
-
Resistance to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis in neuroblastoma cells correlates with a loss of caspase-8 expression.Cancer Res. 2001 Feb 15;61(4):1314-9. Cancer Res. 2001. PMID: 11245427
-
Interleukin-6 sensitizes TNF-α and TRAIL/Apo2L dependent cell death through upregulation of death receptors in human cancer cells.Biochim Biophys Acta Mol Cell Res. 2021 Jun;1868(7):119037. doi: 10.1016/j.bbamcr.2021.119037. Epub 2021 Apr 9. Biochim Biophys Acta Mol Cell Res. 2021. PMID: 33839168
-
Caspase-8 regulation of TRAIL-mediated cell death.Exp Oncol. 2012 Oct;34(3):160-4. Exp Oncol. 2012. PMID: 23070000 Review.
-
Structural determinants of DISC function: new insights into death receptor-mediated apoptosis signalling.Pharmacol Ther. 2013 Nov;140(2):186-99. doi: 10.1016/j.pharmthera.2013.06.009. Epub 2013 Jul 8. Pharmacol Ther. 2013. PMID: 23845861 Review.
Cited by
-
Therapeutic approaches targeting CD95L/CD95 signaling in cancer and autoimmune diseases.Cell Death Dis. 2022 Mar 17;13(3):248. doi: 10.1038/s41419-022-04688-x. Cell Death Dis. 2022. PMID: 35301281 Free PMC article. Review.
-
Onto better TRAILs for cancer treatment.Cell Death Differ. 2016 May;23(5):733-47. doi: 10.1038/cdd.2015.174. Epub 2016 Mar 4. Cell Death Differ. 2016. PMID: 26943322 Free PMC article. Review.
-
Wild-type ALK and activating ALK-R1275Q and ALK-F1174L mutations upregulate Myc and initiate tumor formation in murine neural crest progenitor cells.Oncotarget. 2014 Jun 30;5(12):4452-66. doi: 10.18632/oncotarget.2036. Oncotarget. 2014. PMID: 24947326 Free PMC article.
-
Study of the potential role of CASPASE-10 mutations in the development of autoimmune lymphoproliferative syndrome.Cell Death Dis. 2024 May 4;15(5):315. doi: 10.1038/s41419-024-06679-6. Cell Death Dis. 2024. PMID: 38704374 Free PMC article.
-
Metabolic profiling of human CD4+ cells following treatment with methotrexate and anti-TNF-α infliximab.Cell Cycle. 2013 Sep 15;12(18):3025-36. doi: 10.4161/cc.26067. Epub 2013 Aug 19. Cell Cycle. 2013. PMID: 23974102 Free PMC article.
References
-
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. - PubMed
-
- Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407:770–776. - PubMed
-
- Fulda S. Caspase-8 in cancer biology and therapy. Cancer Lett. 2009;281:128–133. - PubMed
-
- Milhas D, Cuvillier O, Therville N, Clave P, Thomsen M, Levade T, et al. Caspase-10 triggers Bid cleavage and caspase cascade activation in FasL-induced apoptosis. J Biol Chem. 2005;280:19836–19842. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

