Herd immunity to GII.4 noroviruses is supported by outbreak patient sera
- PMID: 19297483
- PMCID: PMC2681945
- DOI: 10.1128/JVI.02518-08
Herd immunity to GII.4 noroviruses is supported by outbreak patient sera
Abstract
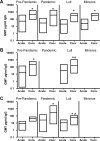
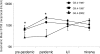
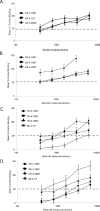
Noroviruses (NoVs) of genogroup II, cluster 4 (GII.4), are the most common cause of outbreaks of acute gastroenteritis worldwide. During the past 13 years, GII.4 NoVs caused four seasons of widespread activity globally, each associated with the emergence of a new strain. In this report, we characterized the most recent epidemic strain, GII.4-2006 Minerva, by comparing virus-like particle (VLP) antigenic relationships and histo-blood group antigen (HBGA) binding profiles with strains isolated earlier. We also investigated the seroprevalence and specificity of GII.4 antibody in the years prior to, during, and following the GII.4 pandemic of 1995 and 1996 using a large collection of acute- and convalescent-phase serum pairs (n = 298) collected from 34 outbreaks. In a surrogate neutralization assay, we measured the blockade of HBGA binding using a panel of GII.4 VLPs representing strains isolated in 1987, 1997, 2002, and 2006 and a GII.3 VLP representing a strain isolated in the mid-1990s. Serum titers required for 50% HBGA blockade were compared between populations. In general, blockade of GII.4 VLP-HBGA binding was greater with convalescent-phase outbreak sera collected near the time of origin of the VLP strain. Heterotypic genotypes did not contribute to herd immunity against GII.4 NoVs based on their inability to block GII.4 VLP binding to HBGA. However, previous exposure to GII.4 NoV followed by infection by GII.3 NoV appeared to evoke an immune response to GII.4 NoV. These results support the hypothesis that herd immunity is a driving force for GII.4 evolution in the U.S. population. The data also suggest that complex patterns of cross-protection may exist across NoV genotypes in humans.
Figures





Similar articles
-
Human Sera Collected between 1979 and 2010 Possess Blocking-Antibody Titers to Pandemic GII.4 Noroviruses Isolated over Three Decades.J Virol. 2017 Jun 26;91(14):e00567-17. doi: 10.1128/JVI.00567-17. Print 2017 Jul 15. J Virol. 2017. PMID: 28468886 Free PMC article.
-
Broad blockade antibody responses in human volunteers after immunization with a multivalent norovirus VLP candidate vaccine: immunological analyses from a phase I clinical trial.PLoS Med. 2015 Mar 24;12(3):e1001807. doi: 10.1371/journal.pmed.1001807. eCollection 2015 Mar. PLoS Med. 2015. PMID: 25803642 Free PMC article. Clinical Trial.
-
Norovirus GII.17 Virus-Like Particles Bind to Different Histo-Blood Group Antigens and Cross-React with Genogroup II-Specific Mouse Sera.Viral Immunol. 2018 Dec;31(10):649-657. doi: 10.1089/vim.2018.0115. Epub 2018 Nov 15. Viral Immunol. 2018. PMID: 30431404
-
Epidemiology of human noroviruses and updates on vaccine development.Curr Opin Gastroenterol. 2014 Jan;30(1):25-33. doi: 10.1097/MOG.0000000000000022. Curr Opin Gastroenterol. 2014. PMID: 24232370 Free PMC article. Review.
-
Norovirus and histo-blood group antigens.Jpn J Infect Dis. 2011;64(2):95-103. Jpn J Infect Dis. 2011. PMID: 21519121 Review.
Cited by
-
Serological surveillance of GI norovirus reveals persistence of blockade antibody in a Jidong community-based prospective cohort, 2014-2018.Front Cell Infect Microbiol. 2023 Dec 18;13:1258550. doi: 10.3389/fcimb.2023.1258550. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 38188632 Free PMC article.
-
Infant antibody and B-cell responses following confirmed pediatric GII.17 norovirus infections functionally distinguish GII.17 genetic clusters.Front Immunol. 2023 Aug 18;14:1229724. doi: 10.3389/fimmu.2023.1229724. eCollection 2023. Front Immunol. 2023. PMID: 37662930 Free PMC article.
-
Emergent variant modeling of the serological repertoire to norovirus in young children.Cell Rep Med. 2023 Mar 21;4(3):100954. doi: 10.1016/j.xcrm.2023.100954. Epub 2023 Feb 27. Cell Rep Med. 2023. PMID: 36854303 Free PMC article.
-
Immune Imprinting Drives Human Norovirus Potential for Global Spread.mBio. 2022 Oct 26;13(5):e0186122. doi: 10.1128/mbio.01861-22. Epub 2022 Sep 14. mBio. 2022. PMID: 36102514 Free PMC article.
-
Controlled Human Infection Models To Accelerate Vaccine Development.Clin Microbiol Rev. 2022 Sep 21;35(3):e0000821. doi: 10.1128/cmr.00008-21. Epub 2022 Jul 6. Clin Microbiol Rev. 2022. PMID: 35862754 Free PMC article. Review.
References
-
- Blanton, L. H., S. M. Adams, R. S. Beard, G. Wei, S. N. Bulens, M. A. Widdowson, R. I. Glass, and S. S. Monroe. 2006. Molecular and epidemiologic trends of caliciviruses associated with outbreaks of acute gastroenteritis in the United States, 2000-2004. J. Infect. Dis. 193413-421. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

