Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability
- PMID: 19240062
- PMCID: PMC2702831
- DOI: 10.1136/gut.2008.165886
Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability
Abstract
Background and aims: Obese and diabetic mice display enhanced intestinal permeability and metabolic endotoxaemia that participate in the occurrence of metabolic disorders. Our recent data support the idea that a selective increase of Bifidobacterium spp. reduces the impact of high-fat diet-induced metabolic endotoxaemia and inflammatory disorders. Here, we hypothesised that prebiotic modulation of gut microbiota lowers intestinal permeability, by a mechanism involving glucagon-like peptide-2 (GLP-2) thereby improving inflammation and metabolic disorders during obesity and diabetes.
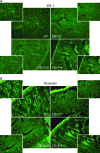
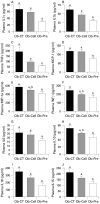
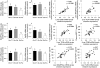
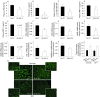
Methods: Study 1: ob/ob mice (Ob-CT) were treated with either prebiotic (Ob-Pre) or non-prebiotic carbohydrates as control (Ob-Cell). Study 2: Ob-CT and Ob-Pre mice were treated with GLP-2 antagonist or saline. Study 3: Ob-CT mice were treated with a GLP-2 agonist or saline. We assessed changes in the gut microbiota, intestinal permeability, gut peptides, intestinal epithelial tight-junction proteins ZO-1 and occludin (qPCR and immunohistochemistry), hepatic and systemic inflammation.
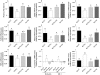
Results: Prebiotic-treated mice exhibited a lower plasma lipopolysaccharide (LPS) and cytokines, and a decreased hepatic expression of inflammatory and oxidative stress markers. This decreased inflammatory tone was associated with a lower intestinal permeability and improved tight-junction integrity compared to controls. Prebiotic increased the endogenous intestinotrophic proglucagon-derived peptide (GLP-2) production whereas the GLP-2 antagonist abolished most of the prebiotic effects. Finally, pharmacological GLP-2 treatment decreased gut permeability, systemic and hepatic inflammatory phenotype associated with obesity to a similar extent as that observed following prebiotic-induced changes in gut microbiota.
Conclusion: We found that a selective gut microbiota change controls and increases endogenous GLP-2 production, and consequently improves gut barrier functions by a GLP-2-dependent mechanism, contributing to the improvement of gut barrier functions during obesity and diabetes.
Conflict of interest statement
Figures


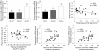
Comment in
-
Probiotic carbohydrates reduce intestinal permeability and inflammation in metabolic diseases.Gut. 2009 Aug;58(8):1044-5. doi: 10.1136/gut.2009.179325. Gut. 2009. PMID: 19592687 No abstract available.
-
Gut microbiota control gut permeability through GLP-2.Gastroenterology. 2010 Feb;138(2):779-81. doi: 10.1053/j.gastro.2009.12.017. Epub 2009 Dec 21. Gastroenterology. 2010. PMID: 20026034 No abstract available.
Similar articles
-
Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice.Diabetes. 2008 Jun;57(6):1470-81. doi: 10.2337/db07-1403. Epub 2008 Feb 27. Diabetes. 2008. PMID: 18305141
-
Responses of gut microbiota and glucose and lipid metabolism to prebiotics in genetic obese and diet-induced leptin-resistant mice.Diabetes. 2011 Nov;60(11):2775-86. doi: 10.2337/db11-0227. Epub 2011 Sep 20. Diabetes. 2011. PMID: 21933985 Free PMC article.
-
GLP-2 enhances barrier formation and attenuates TNFα-induced changes in a Caco-2 cell model of the intestinal barrier.Regul Pept. 2012 Oct 10;178(1-3):95-101. doi: 10.1016/j.regpep.2012.07.002. Epub 2012 Jul 15. Regul Pept. 2012. PMID: 22809889
-
Gut microbiota, intestinal permeability, obesity-induced inflammation, and liver injury.JPEN J Parenter Enteral Nutr. 2011 Sep;35(5 Suppl):14S-20S. doi: 10.1177/0148607111413772. Epub 2011 Aug 1. JPEN J Parenter Enteral Nutr. 2011. PMID: 21807932 Review.
-
The gut microbiome as therapeutic target.Pharmacol Ther. 2011 May;130(2):202-12. doi: 10.1016/j.pharmthera.2011.01.012. Epub 2011 Feb 2. Pharmacol Ther. 2011. PMID: 21295072 Review.
Cited by
-
Gut microbiota metabolites: potential therapeutic targets for Alzheimer's disease?Front Pharmacol. 2024 Sep 17;15:1459655. doi: 10.3389/fphar.2024.1459655. eCollection 2024. Front Pharmacol. 2024. PMID: 39355779 Free PMC article. Review.
-
The Possible Involvement of Glucagon-like Peptide-2 in the Regulation of Food Intake through the Gut-Brain Axis.Nutrients. 2024 Sep 11;16(18):3069. doi: 10.3390/nu16183069. Nutrients. 2024. PMID: 39339669 Free PMC article. Review.
-
What defines a healthy gut microbiome?Gut. 2024 Oct 7;73(11):1893-1908. doi: 10.1136/gutjnl-2024-333378. Gut. 2024. PMID: 39322314 Free PMC article. Review.
-
The role of probiotics on the roadmap to a healthy microbiota: a symposium report.Gut Microbiome (Camb). 2020 Aug 26;1:e2. doi: 10.1017/gmb.2020.2. eCollection 2020. Gut Microbiome (Camb). 2020. PMID: 39296722 Free PMC article. Review.
-
The associations of butyrate-producing bacteria of the gut microbiome with diet quality and muscle health.Gut Microbiome (Camb). 2021 Aug 13;2:e2. doi: 10.1017/gmb.2021.2. eCollection 2021. Gut Microbiome (Camb). 2021. PMID: 39296318 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
