COMMD1 forms oligomeric complexes targeted to the endocytic membranes via specific interactions with phosphatidylinositol 4,5-bisphosphate
- PMID: 18940794
- PMCID: PMC2610505
- DOI: 10.1074/jbc.M804766200
COMMD1 forms oligomeric complexes targeted to the endocytic membranes via specific interactions with phosphatidylinositol 4,5-bisphosphate
Abstract
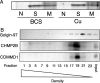
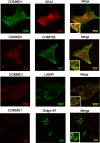
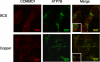
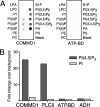
Copper metabolism Murr1 domain 1 (COMMD1) is a 21-kDa protein involved in copper export from the liver, NF-kappaB signaling, HIV infection, and sodium transport. The precise function of COMMD and the mechanism through which COMMD1 performs its multiple roles are not understood. Recombinant COMMD1 is a soluble protein, yet in cells COMMD1 is largely seen as targeted to cellular membranes. Using co-localization with organelle markers and cell fractionation, we determined that COMMD1 is located in the vesicles of the endocytic pathway, whereas little COMMD1 is detected in either the trans-Golgi network or lysosomes. The mechanism of COMMD1 recruitment to cell membranes was investigated using lipid-spotted arrays and liposomes. COMMD1 specifically binds phosphatidylinositol 4,5-bisphosphate (PtdIns(4,5)P2) in the absence of other proteins and does not bind structural lipids; the phosphorylation of PtdIns at position 4 is essential for COMMD1 binding. Proteolytic sensitivity and molecular modeling experiments identified two distinct domains in the structure of COMMD1. The C-terminal domain appears sufficient for lipid binding, because both the full-length and C-terminal domain proteins bind to PtdIns(4,5)P2. In native conditions, endogenous COMMD1 forms large oligomeric complexes both in the cytosol and at the membrane; interaction with PtdIns(4,5)P2 increases the stability of oligomers. Altogether, our results suggest that COMMD1 is a scaffold protein in a distinct sub-compartment of endocytic pathway and offer first clues to its role as a regulator of structurally unrelated membrane transporters.
Figures



Similar articles
-
COMMD1 and PtdIns(4,5)P2 interaction maintain ATP7B copper transporter trafficking fidelity in HepG2 cells.J Cell Sci. 2019 Oct 9;132(19):jcs231753. doi: 10.1242/jcs.231753. J Cell Sci. 2019. PMID: 31515276 Free PMC article.
-
Characterization and copper binding properties of human COMMD1 (MURR1).Biochemistry. 2007 Mar 20;46(11):3116-28. doi: 10.1021/bi0620656. Epub 2007 Feb 20. Biochemistry. 2007. PMID: 17309234
-
Solution structure of the COMMD1 N-terminal domain.J Mol Biol. 2007 Jan 19;365(3):715-21. doi: 10.1016/j.jmb.2006.10.030. Epub 2006 Oct 13. J Mol Biol. 2007. PMID: 17097678 Free PMC article.
-
The many faces of the copper metabolism protein MURR1/COMMD1.J Hered. 2005;96(7):803-11. doi: 10.1093/jhered/esi110. Epub 2005 Nov 2. J Hered. 2005. PMID: 16267171 Review.
-
Regulation of endocytosis by phosphatidylinositol 4,5-bisphosphate and ENTH proteins.Curr Top Microbiol Immunol. 2004;282:31-47. doi: 10.1007/978-3-642-18805-3_2. Curr Top Microbiol Immunol. 2004. PMID: 14594213 Review.
Cited by
-
COMMD1 interacts with the COOH terminus of NKCC1 in Calu-3 airway epithelial cells to modulate NKCC1 ubiquitination.Am J Physiol Cell Physiol. 2013 Jul 15;305(2):C133-46. doi: 10.1152/ajpcell.00394.2012. Epub 2013 Mar 20. Am J Physiol Cell Physiol. 2013. PMID: 23515529 Free PMC article.
-
Does Subtelomeric Position of COMMD5 Influence Cancer Progression?Front Oncol. 2021 Mar 9;11:642130. doi: 10.3389/fonc.2021.642130. eCollection 2021. Front Oncol. 2021. PMID: 33768002 Free PMC article.
-
Liver-specific Commd1 knockout mice are susceptible to hepatic copper accumulation.PLoS One. 2011;6(12):e29183. doi: 10.1371/journal.pone.0029183. Epub 2011 Dec 22. PLoS One. 2011. PMID: 22216203 Free PMC article.
-
COMMD1 and PtdIns(4,5)P2 interaction maintain ATP7B copper transporter trafficking fidelity in HepG2 cells.J Cell Sci. 2019 Oct 9;132(19):jcs231753. doi: 10.1242/jcs.231753. J Cell Sci. 2019. PMID: 31515276 Free PMC article.
-
Cell Penetrating Capacity and Internalization Mechanisms Used by the Synthetic Peptide CIGB-552 and Its Relationship with Tumor Cell Line Sensitivity.Molecules. 2018 Mar 30;23(4):801. doi: 10.3390/molecules23040801. Molecules. 2018. PMID: 29601540 Free PMC article.
References
-
- van De Sluis, B., Rothuizen, J., Pearson, P. L., van Oost, B. A., and Wijmenga, C. (2002) Hum. Mol. Genet. 11 165-173 - PubMed
-
- Klomp, A. E., van de Sluis, B., Klomp, L. W., and Wijmenga, C. (2003) J. Hepatol. 39 703-709 - PubMed
-
- Kawamura, M., Takahashi, I., and Kaneko, J. J. (2002) Vet. Pathol. 39 747-750 - PubMed
-
- Narindrasorasak, S., Kulkarni, P., Deschamps, P., She, Y. M., and Sarkar, B. (2007) Biochemistry 46 3116-3128 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

