Solution structure of the COMMD1 N-terminal domain
- PMID: 17097678
- PMCID: PMC2706016
- DOI: 10.1016/j.jmb.2006.10.030
Solution structure of the COMMD1 N-terminal domain
Abstract
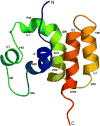
COMMD1 is the prototype of a new protein family that plays a role in several important cellular processes, including NF-kappaB signaling, sodium transport, and copper metabolism. The COMMD proteins interact with one another via a conserved C-terminal domain, whereas distinct functions are predicted to result from a variable N-terminal domain. The COMMD proteins have not been characterized biochemically or structurally. Here, we present the solution structure of the N-terminal domain of COMMD1 (N-COMMD1, residues 1-108). This domain adopts an alpha-helical structure that bears little resemblance to any other helical protein. The compact nature of N-COMMD1 suggests that full-length COMMD proteins are modular, consistent with specific functional properties for each domain. Interactions between N-COMMD1 and partner proteins may occur via complementary electrostatic surfaces. These data provide a new foundation for biochemical characterization of COMMD proteins and for probing COMMD1 protein-protein interactions at the molecular level.
Figures
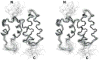


Similar articles
-
Characterization of COMMD protein-protein interactions in NF-kappaB signalling.Biochem J. 2006 Aug 15;398(1):63-71. doi: 10.1042/BJ20051664. Biochem J. 2006. PMID: 16573520 Free PMC article.
-
Characterization and copper binding properties of human COMMD1 (MURR1).Biochemistry. 2007 Mar 20;46(11):3116-28. doi: 10.1021/bi0620656. Epub 2007 Feb 20. Biochemistry. 2007. PMID: 17309234
-
COMMD1 forms oligomeric complexes targeted to the endocytic membranes via specific interactions with phosphatidylinositol 4,5-bisphosphate.J Biol Chem. 2009 Jan 2;284(1):696-707. doi: 10.1074/jbc.M804766200. Epub 2008 Oct 21. J Biol Chem. 2009. PMID: 18940794 Free PMC article.
-
COMMD proteins and the control of the NF kappa B pathway.Cell Cycle. 2007 Mar 15;6(6):672-6. doi: 10.4161/cc.6.6.3989. Epub 2007 Mar 7. Cell Cycle. 2007. PMID: 17361106 Free PMC article. Review.
-
COMMD1: A Multifunctional Regulatory Protein.J Cell Biochem. 2018 Jan;119(1):34-51. doi: 10.1002/jcb.26151. Epub 2017 Jun 13. J Cell Biochem. 2018. PMID: 28543362 Review.
Cited by
-
COMMD1 interacts with the COOH terminus of NKCC1 in Calu-3 airway epithelial cells to modulate NKCC1 ubiquitination.Am J Physiol Cell Physiol. 2013 Jul 15;305(2):C133-46. doi: 10.1152/ajpcell.00394.2012. Epub 2013 Mar 20. Am J Physiol Cell Physiol. 2013. PMID: 23515529 Free PMC article.
-
Structural Organization of the Retriever-CCC Endosomal Recycling Complex.Res Sq [Preprint]. 2023 Jun 16:rs.3.rs-3026818. doi: 10.21203/rs.3.rs-3026818/v1. Res Sq. 2023. Update in: Nat Struct Mol Biol. 2024 Jun;31(6):910-924. doi: 10.1038/s41594-023-01184-4 PMID: 37397996 Free PMC article. Updated. Preprint.
-
Systems-wide Studies Uncover Commander, a Multiprotein Complex Essential to Human Development.Cell Syst. 2017 May 24;4(5):483-494. doi: 10.1016/j.cels.2017.04.006. Cell Syst. 2017. PMID: 28544880 Free PMC article. Review.
-
Structural Organization of the Retriever-CCC Endosomal Recycling Complex.bioRxiv [Preprint]. 2023 Jun 7:2023.06.06.543888. doi: 10.1101/2023.06.06.543888. bioRxiv. 2023. Update in: Nat Struct Mol Biol. 2024 Jun;31(6):910-924. doi: 10.1038/s41594-023-01184-4 PMID: 37333304 Free PMC article. Updated. Preprint.
-
Structural insights into the architecture and membrane interactions of the conserved COMMD proteins.Elife. 2018 Aug 1;7:e35898. doi: 10.7554/eLife.35898. Elife. 2018. PMID: 30067224 Free PMC article.
References
-
- Van De Sluis B, Rothuizen J, Pearson PL, Van Oost BA, Wijmenga C. Identification of a new copper metabolism gene by positional cloning in a purebred dog population. Hum Mol Genet. 2002;11:165–173. - PubMed
-
- Tao TY, Liu F, Klomp L, Wijmenga C, Gitlin JD. The copper toxicosis gene product Murr1 interacts directly with the Wilson disease protein. J Biol Chem. 2003;278:41593–41596. - PubMed
-
- Klomp AEM, Van De Sluis B, Klomp LWJ, Wijmenga C. The ubiquitously expressed Murr1 protein is absent in canine copper toxicosis. J Hepatol. 2003;39:703–709. - PubMed
-
- Hyun C, Filippich LC. Inherited canine copper toxicosis in Australian Bedlington Terriers. J Vet Sci. 2004;5:19–28. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

