ESPript/ENDscript: Extracting and rendering sequence and 3D information from atomic structures of proteins
- PMID: 12824317
- PMCID: PMC168963
- DOI: 10.1093/nar/gkg556
ESPript/ENDscript: Extracting and rendering sequence and 3D information from atomic structures of proteins
Abstract
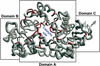
The fortran program ESPript was created in 1993, to display on a PostScript figure multiple sequence alignments adorned with secondary structure elements. A web server was made available in 1999 and ESPript has been linked to three major web tools: ProDom which identifies protein domains, PredictProtein which predicts secondary structure elements and NPS@ which runs sequence alignment programs. A web server named ENDscript was created in 2002 to facilitate the generation of ESPript figures containing a large amount of information. ENDscript uses programs such as BLAST, Clustal and PHYLODENDRON to work on protein sequences and such as DSSP, CNS and MOLSCRIPT to work on protein coordinates. It enables the creation, from a single Protein Data Bank identifier, of a multiple sequence alignment figure adorned with secondary structure elements of each sequence of known 3D structure. Similar 3D structures are superimposed in turn with the program PROFIT and a final figure is drawn with BOBSCRIPT, which shows sequence and structure conservation along the Calpha trace of the query. ESPript and ENDscript are available at http://genopole.toulouse.inra.fr/ESPript.
Figures

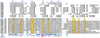
Similar articles
-
ENDscript: a workflow to display sequence and structure information.Bioinformatics. 2002 May;18(5):767-8. doi: 10.1093/bioinformatics/18.5.767. Bioinformatics. 2002. PMID: 12050076
-
Deciphering key features in protein structures with the new ENDscript server.Nucleic Acids Res. 2014 Jul;42(Web Server issue):W320-4. doi: 10.1093/nar/gku316. Epub 2014 Apr 21. Nucleic Acids Res. 2014. PMID: 24753421 Free PMC article.
-
ESPript: analysis of multiple sequence alignments in PostScript.Bioinformatics. 1999 Apr;15(4):305-8. doi: 10.1093/bioinformatics/15.4.305. Bioinformatics. 1999. PMID: 10320398
-
The ProDom database of protein domain families: more emphasis on 3D.Nucleic Acids Res. 2005 Jan 1;33(Database issue):D212-5. doi: 10.1093/nar/gki034. Nucleic Acids Res. 2005. PMID: 15608179 Free PMC article.
-
The PredictProtein server.Nucleic Acids Res. 2003 Jul 1;31(13):3300-4. doi: 10.1093/nar/gkg508. Nucleic Acids Res. 2003. PMID: 12824312 Free PMC article.
Cited by
-
Structures of Brucella ovis leucine-, isoleucine-, valine-, threonine- and alanine-binding protein reveal a conformationally flexible peptide-binding cavity.Acta Crystallogr F Struct Biol Commun. 2024 Sep 1;80(Pt 9):193-199. doi: 10.1107/S2053230X24007027. Epub 2024 Aug 23. Acta Crystallogr F Struct Biol Commun. 2024. PMID: 39177244 Free PMC article.
-
In-silico structural analysis of Pseudomonas syringae effector HopZ3 reveals ligand binding activity and virulence function.J Plant Res. 2021 May;134(3):599-611. doi: 10.1007/s10265-021-01274-8. Epub 2021 Mar 17. J Plant Res. 2021. PMID: 33730245
-
Heterozygous reelin mutations cause autosomal-dominant lateral temporal epilepsy.Am J Hum Genet. 2015 Jun 4;96(6):992-1000. doi: 10.1016/j.ajhg.2015.04.020. Am J Hum Genet. 2015. PMID: 26046367 Free PMC article.
-
Crystal structure of the pilotin from the enterohemorrhagic Escherichia coli type II secretion system.J Struct Biol. 2013 May;182(2):186-91. doi: 10.1016/j.jsb.2013.02.013. Epub 2013 Feb 28. J Struct Biol. 2013. PMID: 23458689 Free PMC article.
-
Characterization of a Highly pH Stable Chi-Class Glutathione S-Transferase from Synechocystis PCC 6803.PLoS One. 2015 May 12;10(5):e0126811. doi: 10.1371/journal.pone.0126811. eCollection 2015. PLoS One. 2015. PMID: 25965384 Free PMC article.
References
-
- Chothia C. and Lesk,A.M. (1980) How different amino acid sequences determine similar protein structures: the structure and evolutionary dynamics of the globin. J. Mol. Biol., 136, 225–270. - PubMed
-
- Gouet P., Courcelle,E., Stuart,D.I. and Metoz,F. (1999) ESPript: multiple sequence alignments in PostScript. Bioinformatics, 15, 305–308. - PubMed
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

