Disease flares with baricitinib dose reductions and development of flare criteria in patients with CANDLE/PRAAS
- PMID: 38653530
- PMCID: PMC11420725
- DOI: 10.1136/ard-2023-225463
Disease flares with baricitinib dose reductions and development of flare criteria in patients with CANDLE/PRAAS
Abstract
Objectives: Patients with chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperature/proteasome-associated autoinflammatory syndrome (CANDLE/PRAAS) respond to the janus kinase inhibitor 1/2 inhibition with baricitinib at exposures higher than in rheumatoid arthritis. Baricitinib dose reductions to minimise exposure triggered disease flares which we used to develop 'flare criteria'.
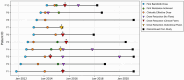
Methods: Of 10 patients with CANDLE/PRAAS treated with baricitinib in an open-label expanded-access programme, baricitinib doses were reduced 14 times in 9 patients between April 2014 and December 2019. Retrospective data analysis of daily diary scores and laboratory markers collected before and after the dose reductions were used to develop 'clinical' and 'subclinical' flare criteria. Disease flare rates were compared among patients with <25% and >25% dose reductions and during study visits when patients received recommended 'optimized' baricitinib doses (high-dose visits) versus lower than recommended baricitinib doses (low-dose visits) using two-sided χ2 tests.
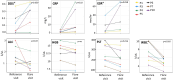
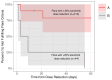
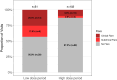
Results: In the 9/10 patients with CANDLE with dose reduction, 7/14 (50%) times the dose was reduced resulted in a disease flare. All four dose reductions of >25% triggered a disease flare (p <0.05). Assessment of clinical and laboratory changes during disease flares allowed the development of disease flare criteria that were assessed during visits when patients received high or low doses of baricitinib. Disease flare criteria were reached during 43.14% of low-dose visits compared with 12.75% of high-dose visits (p <0.0001). Addition of an interferon score as an additional flare criterion increased the sensitivity to detect disease flares.
Conclusion: We observed disease flares and rebound inflammation with baricitinib dose reductions and proposed flare criteria that can assist in monitoring disease activity and in designing clinical studies in CANDLE/PRAAS.
Keywords: Immune System Diseases; Inflammation; Outcome Assessment, Health Care.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY. Published by BMJ on behalf of EULAR.
Conflict of interest statement
Competing interests: PAB received consulting and lecture fees and travel support from SOBI, lecture fees from Novartis and serves as a trustee of society, a patient led Kawasaki charity (unpaid). SM received lecture and presentation fees and travel support from Abbvie, Novartis, Roche, Pfizer. SS received lecture fee from Novartis and presentation fee from Pfizer. RG-M received study support under government CRADAs from Eli Lilly, IFM and SOBI and serves in a leadership position at the TARN initiative and as liaison to the Autoinflammatory Alliance (unpaid).
Figures
Similar articles
-
JAK1/2 inhibition with baricitinib in the treatment of autoinflammatory interferonopathies.J Clin Invest. 2018 Jul 2;128(7):3041-3052. doi: 10.1172/JCI98814. Epub 2018 Jun 11. J Clin Invest. 2018. PMID: 29649002 Free PMC article.
-
Evaluation of hepatitis B virus in clinical trials of baricitinib in rheumatoid arthritis.RMD Open. 2020 Feb;6(1):e001095. doi: 10.1136/rmdopen-2019-001095. RMD Open. 2020. PMID: 32098857 Free PMC article. Clinical Trial.
-
Efficacy and safety of baricitinib in Japanese patients with autoinflammatory type I interferonopathies (NNS/CANDLE, SAVI, And AGS).Pediatr Rheumatol Online J. 2023 Apr 22;21(1):38. doi: 10.1186/s12969-023-00817-8. Pediatr Rheumatol Online J. 2023. PMID: 37087470 Free PMC article. Clinical Trial.
-
The safety of baricitinib in patients with rheumatoid arthritis.Expert Opin Drug Saf. 2020 May;19(5):545-551. doi: 10.1080/14740338.2020.1743263. Epub 2020 Mar 21. Expert Opin Drug Saf. 2020. PMID: 32174196 Review.
-
Baricitinib: The Second FDA-Approved JAK Inhibitor for the Treatment of Rheumatoid Arthritis.Ann Pharmacother. 2019 Sep;53(9):947-953. doi: 10.1177/1060028019839650. Epub 2019 Mar 24. Ann Pharmacother. 2019. PMID: 30907116 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
