Abstract
Background
Long-acting cabotegravir (CAB-LA) is highly effective for HIV prevention, but delayed HIV diagnoses and integrase strand transfer inhibitor (INSTI) resistance were observed in trials. We report the first case in routine clinical care of HIV infection on CAB-LA with INSTI resistance.
Methods
The SeroPrEP study enrolls individuals in the United States who acquire HIV on pre-exposure prophylaxis modalities to assess diagnostics, antiretroviral (ARV) drug levels, resistance, and treatment outcomes. Resistance mutations in full-length HIV-1 integrase were identified by single-genome sequencing (SGS). Cabotegravir concentrations in plasma and hair segments were measured by liquid chromatography–tandem mass spectrometry.
Results
A 23-year-old gender-nonbinary person, male at birth, restarted CAB-LA 6 months after discontinuation due to losing insurance. Prior to restart, HIV-1 RNA was not detected, but 20 days elapsed before CAB-LA injection. After the second CAB-LA injection, HIV antigen/antibody returned reactive (HIV-1 RNA 451 copies/mL). SGS of plasma HIV-1 RNA identified INSTI mutation Q148R in 2/24 sequences 2 days postdiagnosis; commercial genotype failed amplification. Cabotegravir hair concentration was 0.190 ng/mg 2 weeks prediagnosis; plasma cabotegravir was high (3.37 μg/mL; ∼20× PA-IC90) 14 days postdiagnosis. Viral suppression was maintained for 6 months on darunavir/cobicistat/emtricitabine/tenofovir alafenamide, then switched to doravirine + emtricitabine/tenofovir alafenamide due to nausea.
Conclusions
In this first case of HIV infection on CAB-LA with INSTI resistance in routine care, cabotegravir resistance was detected only with a sensitive research assay. Accelerated pathways to minimize time between HIV testing and CAB-LA initiation are needed to optimize acute HIV detection and mitigate resistance risk. Sustained product access regardless of insurance is imperative to reduce HIV infections on CAB-LA.
Keywords: breakthrough infection, HIV prevention, pharmacokinetics, pre-exposure prophylaxis, resistance
Long-acting injectable cabotegravir (CAB-LA) is a highly effective HIV prevention strategy and was superior to oral pre-exposure prophylaxis (PrEP) with daily tenofovir disoproxil fumarate/emtricitabine (TDF/FTC) in registrational trials among men and transgender women who have sex with men and cisgender women [1–3]. CAB-LA was approved by the US Food and Drug Administration in 2021 and endorsed by the World Health Organization (WHO) in 2022 [4].
Although CAB-LA is highly effective, rare cases of HIV infection have occurred in trials [5, 6]. Incident cases of HIV infection were characterized by delayed detection of infection (false-negative fourth-generation antigen/antibody [Ag/Ab] assays), resulting in accumulation of integrase strand transfer inhibitor (INSTI) resistance mutations in the setting of cabotegravir exposure. In addition, INSTI resistance emerged among some participants who started or restarted CAB-LA in the setting of undetected prevalent HIV infection. Due to cross-resistance between CAB and HIV treatment, INSTI resistance may impact antiretroviral therapy (ART) options and success, particularly in settings where dolutegravir-based ART is first-line and access to both HIV drug resistance testing and protease inhibitors is limited [7].
As CAB-LA access expands, cases of breakthrough HIV infection are expected to remain rare—with 1 case published to date [8]—but have significant potential consequences. CAB-LA implementation in routine clinical care differs from registrational trials in terms of HIV testing (including frequency of monitoring and sensitivity of diagnostic assays used before CAB-LA start and during follow-up) and use of a direct-to-inject strategy for CAB-LA injections without oral lead-in. Moreover, in the United States, insurance approval and procurement of CAB-LA on-site in clinics may result in delays between HIV testing and CAB-LA injections that do not occur in trials. Delays in detection of HIV infection using standard diagnostic algorithms may occur, and resistance assays may fail because of suppression of viremia to below the detection limit of standard methods [9], leading to lack of information on INSTI resistance to guide treatment decisions in routine care.
As the use of CAB-LA for prevention increases, systems are needed to detect HIV infections and comprehensively assess diagnostics, drug levels, resistance, and HIV treatment outcomes following ART initiation. We led a study to assess such outcomes and report here the first case in routine care of acute HIV infection with INSTI resistance in an individual using CAB-LA.
METHODS
A 23-year-old gender-nonbinary individual, assigned male at birth, initially started oral tenofovir-based PrEP 4 years before HIV diagnosis. The patient had a gap in health insurance coverage and presented to a clinic in California to restart PrEP 15 months before HIV diagnosis. They requested to switch to injectable PrEP due to pill-taking barriers, including substance use. HIV RNA was not detected (ND) in plasma (Hologic Aptima Quant Dx), and daily oral FTC/TAF was started pending CAB-LA availability (Figure 1). Five weeks later (day −399), the patient received CAB-LA 600 mg IM; HIV RNA was ND. They received 4 additional CAB-LA injections as scheduled, except 1 injection 7 days after the scheduled date (covered by oral FTC/TAF). After the fifth injection, the patient experienced another loss of insurance and care interruption.
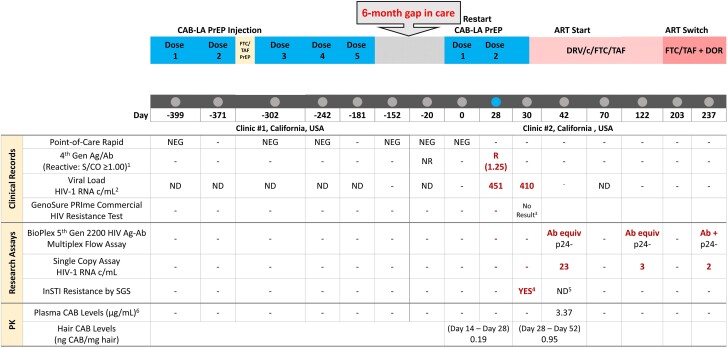
Figure 1.
Timeline of ARV administration and research and clinical testing for HIV diagnosis, PK, and ARV resistance. 1. ARCHITECT HIV Ag/Ab Combo; signal to cutoff value ≥1.00 considered reactive. 2. Clinic #1: Hologic Aptima HIV Quant Dx Assay, LOQ 30 copies/mL; Clinic #2: COBAS AmpliPrep/COBAS TaqMan HIV-1 Test, version 2.0; LOQ (limit of quantitation) 20 copies/mL. 3. No result due to failed PCR amplification in the commercial HIV genotyping assay. 4. Q148R was detected at 8% frequency and A128T was detected at 4% frequency on separate genomes. Full genotype is presented in Table 1. 5. Day 42 SGS limited in sensitivity to detect resistance due to HIV-1 RNA 23 copies/mL and only 3 genomes sequenced. 6. Plasma CAB concentration on day 42 (14 days after second injection) was ∼20× PA-IC90 (well above 8× PA-IC90). Abbreviations: Ag/Ab, antigen/antibody; ART, antiretroviral therapy; ARV, antiretroviral; CAB, cabotegravir; CAB-LA, long-acting cabotegravir; DOR, doravirine; DRV/c/FTC/TAF, darunavir/cobicistat/emtricitabine/tenofovir alafenamide; equiv, equivocal; FTC/TAF, emtricitabine/tenofovir alafenamide; INSTI, integrase strand transfer inhibitor; ND, not detected; NR, nonreactive; PK, pharmacokinetics; PrEP, pre-exposure prophylaxis; R, reactive; S/CO, signal to cutoff; SGS, single-genome sequencing.
Six months later, the patient presented to a second clinic to restart CAB-LA. Their body mass index was 18 kg/m2; they were taking no medications. Before CAB-LA restart (161 days after CAB-LA dose 5), HIV-1 p24 Ag and HIV-1/HIV-2 Ab were nonreactive (NR; ARCHITECT HIV Ag/Ab Combo); HIV-1 RNA target not detected (TND; COBAS AmpliPrep/COBAS TaqMan HIV-1 Test). Chlamydia, gonorrhea, and syphilis tests were negative. Twenty days elapsed between diagnostic screening and injection, while awaiting CAB-LA and the patient's return to the clinic. On day 0 (Figure 1), the patient restarted CAB-LA. Point-of-care (POC) HIV antibody was negative; laboratory-based testing was not repeated.
On day 28, blood was drawn for standard laboratory-based testing, and a second CAB-LA injection was administered. POC testing was not performed. Following the injection, labs returned with HIV Ag/Ab reactive (ARCHITECT; signal-to-cutoff [S/CO] value 1.24; S/CO ≥1.00 considered reactive; HIV-1 RNA 451 copies/mL). The patient returned 2 days later (day 30) for additional testing: CD4 cell count was 810 cells/μL (35%); HIV-1 RNA was 410 c/mL. A sample sent to a commercial laboratory for genotyping failed to amplify.
At the time of HIV diagnosis, the patient initially reported no acute HIV symptoms, but they later recalled having experienced subjective fevers, chills, and headache ∼8 days after CAB-LA restart; they were unsure if symptoms were related to drug use. They reported several psychosocial barriers to care, including unemployment, unstable housing, moderate depression (Patient Health Questionnaire-9 [PHQ-9] = 14), heavy alcohol use (Alcohol Use Disorders Identification Test - Consumption [AUDIT-C] = 11) [10], and poly-substance use (no injection drug use). They had had 6 male sex partners (receptive and insertive condomless anal intercourse) in the 3 previous months but were not aware of any partners with HIV.
On day 34, ART was started with a single tablet of darunavir/cobicistat/emtricitabine/tenofovir alafenamide (DRV/c/FTC/TAF) once daily. In accordance with standard health department protocols, the patient was contacted by local health department staff to support linkage to care, HIV test result disclosure, and partner services.
The SeroPrEP Study
The SeroPrEP study (seroprep.ucsf.edu) examines breakthrough HIV infections in patients on oral and long-acting PrEP in the United States [11–14], with referrals through the National Clinician Consultation Center (NCCC) PrEPline and Warmline [15]. As recommended in the US Centers for Disease Control and Prevention (CDC) PrEP Clinical Practice Guidelines [16], upon receipt of reactive HIV test results, the patient's provider called the NCCC, which provides clinical consultation on HIV testing and management for potential HIV acquisition on PrEP. Through an institutional review board–approved study protocol, the NCCC referred the provider to the SeroPrEP study.
The patient provided written informed consent to enroll in SeroPrEP, which is approved by the UCSF Committee on Human Research. After enrollment, blood was collected for confirmatory diagnostics, genotypic HIV resistance testing, and pharmacokinetics (PK). Remnant plasma samples from clinical care of the participant and medical records were obtained. The participant underwent follow-up visits to assess HIV treatment outcomes after ART initiation. De-identified information on partner HIV testing from the local health department was obtained with permission. A behavioral questionnaire was administered to assess demographics, sexual behavior, substance use, and mental health.
For virologic testing, HIV-1 RNA below routine thresholds was quantified using an automated single copy assay (autoSCA) that detects HIV-1 RNA down to ≤1 copies/mL [17]. HIV antigen/antibody reactivity was verified using a fifth-generation assay (Bioplex 2200 HIV Ag/Ab Multiplex Flow). INSTI mutations in plasma HIV RNA were identified by single-genome sequencing (SGS) of full-length integrase, as described previously [9]. Testing was performed at the University of Pittsburgh Virology Specialty Laboratory (Pittsburgh, PA, USA).
For pharmacokinetic testing, CAB concentrations were quantified in plasma and segmented hair via liquid chromatography–tandem mass spectrometry using validated methods [18]. The plasma assay has a linear dynamic range of 0.0100–20.0 µg/mL, with a lower limit of quantitation (LLOQ) of 0.0100 µg/mL. The hair CAB assay has a linear dynamic range of 0.00625–3.20 ng/mg, with an LLOQ of 0.00625 ng/mg. Testing was performed at the UCSF Hair Analytical Laboratory (San Francisco, CA, USA).
RESULTS
After the participant enrolled in SeroPrEP, we performed sensitive research assays for detection of INSTI resistance mutations and pharmacokinetic exposure to CAB and followed the participant for 6 months to assess virologic outcomes on ART.
Integrase SGS was performed on plasma collected from the participant 2 days after testing HIV Ag/Ab reactive (ARCHITECT) and receipt of second CAB-LA injection (day 30). HIV-1 RNA in this sample was 410 c/mL. Integrase SGS identified the mutations Q148R (an INSTI resistance-associated mutation [RAM] selected by CAB) in 2/24 sequences and A128T (a nonpolymorphic INSTI accessory mutation) in 1/24 sequences (Table 1).
Table 1.
Plasma Genotype by Single-Genome Sequencing
| Mutation Frequency (%)a | Genotype (Full-Length Integrase)b | Interpretation |
|---|---|---|
| Plasma genotype by SGS: sample collected on day 30 (2 d after first reactive HIV test and before ART start) | ||
| 19/24 (67) | L45Q, G59E, I72V, L101I, V201I, D270N | No INSTI resistance |
| 2/24 (8) | L45Q, G59E, I72V, L101I, Q148R, V201I, D270N | Q148R is a major INSTI resistance-associated mutationc |
| 1/24 (4) | L45Q, G59E, I72V, L101I, A128T, V201I, D270N | A128T is a nonpolymorphic INSTI accessory mutation |
| 1/24 (4) | L45Q, G59E, I72V, G94R, L101I, V201I, D270N | No INSTI resistance |
| 1/24 (4) | L45Q, G59E, W61*, I72V, L101I, V201I, D270N | Contains stop codon (*); likely nonviable virus |
| Plasma genotype by SGS: sample collected on day 42 (14 d after first reactive HIV test and 8 d after ART start) | ||
| 3/3 (100) | L45Q, G59E, I72V, L101I, V201I, D270N | No INSTI resistance |
Abbreviations: ART, antiretroviral therapy; CAB, cabotegravir; INSTI, integrase strand transfer inhibitor; RAM, resistance-associated mutation; SGS, single-genome sequencing.
aMutation frequency calculated as number of genomes with reported genotype as a proportion of number of genomes sequenced. From the day 30 sample, 24 genomes were sequenced, yielding a 90% certainty that a mutation present at 10% frequency would be detected. From the day 42 sample, 3 genomes were sequenced, yielding a 90% certainty that a mutation present at 50% frequency would be detected.
bMutations were defined using Stanford HIVDB, version 9.5.1, using an HXB2 subtype B reference. Major drug resistance-associated mutations are indicated in bold text, accessory mutations are indicated in underlined text, and integrase gene polymorphisms are indicated in black text.
cQ148R confers high-level resistance to cabotegravir and low-level resistance to dolutegravir and bictegravir. Although Q148R alone may not affect response to INSTIs used for first-line HIV treatment, intermediate or high-level INSTI resistance can occur when Q148R is present in combination with other INSTI RAMs (eg, if additional INSTI RAMs accumulate in the setting of cabotegravir exposure before HIV detection).
Plasma from day 42 (14 days postdiagnosis, 8 days following ART start) was HIV-1 Ab equivocal/HIV-1 Ag nonreactive (BioPlex 2200). Integrase SGS showed no mutations in 3/3 sequences in the setting of low-level viremia (HIV-1 RNA 23 copies/mL by autoSCA). Given that only 3 genomes could be sequenced, there was limited sensitivity to detect INSTI resistance mutations at low frequency in the virus population in this sample.
Pharmacokinetic testing was performed on plasma from day 42; the CAB concentration was 3.37 μg/mL (∼20× PA-IC90, with 8× PA-IC90 associated with high concentrations of CAB in trials). A hair sample collected on day 52 was analyzed in segments reflecting ∼2 weeks before and 3 weeks after HIV diagnosis and the second CAB-LA injection: CAB concentrations were 0.190 ng/mg and 0.950 ng/mg, respectively (within the range of observed concentrations with CAB for treatment) [18].
Through routine HIV partner services with the local public health department, the patient named several recent sexual partners. The likely partner had a recent HIV RNA of ∼5000 copies/mL. This partner's plasma HIV genotype obtained using a commercial next-generation sequencing (NGS) assay (GenoSure PRime, Monogram) did not have INSTI resistance mutations.
The participant has remained engaged in HIV care and treatment through 6 months after their HIV diagnosis. One month after ART initiation with DRV/c/FTC/TAF (day 70), plasma HIV-RNA was TND. The participant initiated gender-affirming hormone therapy (GAHT) with spironolactone and estradiol at this visit. The participant experienced nausea requiring the use of daily oral antiemetic medication. Five months after HIV diagnosis (day 203), the participant switched to once-daily FTC/TAF and doravirine and stopped GAHT; the nausea subsided. Subsequent HIV-1 RNA was 2.0 c/mL (autoSCA) on day 237.
DISCUSSION
We report the first case, to our knowledge, of HIV seroconversion with INSTI resistance in an individual receiving CAB-LA PrEP in routine clinical care. INSTI resistance was detected within 30 days of CAB-LA restart and required a sensitive research assay for detection. This case highlights the need for optimal testing strategies to detect acute HIV infection before CAB-LA start and during ongoing injections to mitigate the risk of INSTI resistance [9]. Moreover, accelerated pathways are needed to minimize time between planned CAB-LA start, HIV testing, and initial injection and to ensure uninterrupted access to CAB-LA regardless of insurance status.
CAB-LA PrEP access has been gradually increasing in the United States and Europe and is expected to expand globally in coming years. Given the remarkable efficacy of CAB-LA, cases of HIV acquisition should remain rare among persons using CAB-LA PrEP in routine care but merit investigation due to significant potential consequences in terms of cross-resistance to INSTI-based ART regimens, which are first-line globally [19]. As CAB-LA is scaled up, monitoring is needed to identify cases of HIV acquisition in patients on CAB-LA and evaluate the pharmacokinetics, resistance, and subsequent outcomes on ART.
HIV diagnostic approaches, including optimal assays to detect acute HIV infection and mitigate resistance risk [9], remain an open and important question during CAB-LA rollout. In this case, HIV acquisition likely occurred shortly before or around the time of CAB-LA restart. At the time of CAB-LA restart, the POC HIV antibody test was negative; HIV-1 RNA was ND 20 days earlier. HIV infection therefore could have occurred as early as ∼30 days before CAB-LA restart (given the ∼10 day period for the HIV-1 RNA assay) or around the time of CAB-LA restart. The CDC 2021 guidelines recommend HIV-1 RNA testing ideally within 1 week before CAB-LA start and at each visit [16], although fidelity to this recommendation is variable in practice. In many global settings, availability of HIV RNA testing with rapid return of results may not be routinely available. The WHO 2022 cabotegravir guidelines state that national HIV testing strategies, including rapid HIV antibody testing and enzyme immunoassays, can be used, with consideration of nucleic acid testing where feasible [4]. Additional data from open-label extensions of HPTN 083/084, early implementation studies, and HIV surveillance may inform testing recommendations [20, 21]. SeroPrEP is also designed to help inform diagnostic strategies.
In routine care, delays in accessing CAB-LA injections may result in ongoing risk of HIV acquisition while awaiting insurance approval and procuring CAB-LA on-site in the clinic. Given the safety of intramuscular CAB in trials, an oral lead-in before first injection is not needed, and a direct-to-inject strategy is being routinely used in clinical settings. However, gaps between planned start (and HIV testing) and first injection can occur. In this case, 20 days elapsed between planned CAB-LA restart and injection, during which time the participant was not using biomedical prevention. During anticipated delays in CAB-LA access, some clinicians are starting oral tenofovir-based PrEP as a bridge to CAB-LA to provide interim HIV protection [22]. Accelerated pathways are needed to minimize time between patients choosing and initiating CAB-LA, ideally with a same-day start.
The INSTI resistance mutation Q148R was detected at low frequency within 30 days of CAB-LA restart by single-genome sequencing, which is a sensitive research assay. Commercial standard genotyping failed in this case, likely due to HIV RNA <500 c/mL, consistent with prior reports [8, 9]. The INSTI mutation Q148R is commonly detected in individuals with virologic failure on cabotegravir/rilpivirine for HIV treatment and HIV infection on CAB-LA PrEP [6, 23]. In HPTN 083, Q148R was detected in 6 participants and Q148K in 1 additional participant. Although Q148R alone may not affect response to INSTIs used for first-line treatment, it can reduce susceptibility to dolutegravir and bictegravir in combination with mutations at codons 140 and 138 in the integrase gene [7]. In this participant, INSTI resistance may have emerged on CAB-LA if the individual restarted CAB during undetected acute HIV infection. INSTI resistance could have been transmitted to the participant from one of their partners, but this possibility is less likely; no INSTI mutations were detected in the presumed partner's genotype using a commercial assay that can detect mutations present at >10% frequency [24], and the prevalence of transmitted INSTI RAMs has been low in the United States to date [25]. With the global use of INSTIs as first-line ART, there are increasing reports of INSTI resistance among people with HIV on treatment, and the potential for transmitted INSTI resistance merits monitoring [26].
As CAB-LA is introduced globally, a major question is the risk of INSTI resistance should HIV infection occur. Among 37 individuals (32 from HPTN 083, 4 from HPTN 084, and the current participant) with HIV infection after receiving CAB for prevention who had successful resistance testing, major INSTI RAMs were identified among 11 (30%) (Supplementary Table) [5, 6]. In registrational trials, accumulation of additional INSTI RAMs over time occurred due to delayed HIV detection. Among 19 individuals with CAB administration within 6 months of the first visit at which HIV was detected (including the current case), 11 (58%) developed major INSTI RAMs. Ongoing monitoring of INSTI resistance following CAB-LA breakthrough is warranted.
Optimal HIV treatment regimens for persons who acquire HIV on CAB-LA have not been defined. This participant started DRV/c/FTC/TAF, as recommended in US Department of Health and Human Services guidelines for initial ART following HIV acquisition on CAB-LA [27]. INSTI-based ART was not used due to concern for the risk of INSTI resistance. Viral suppression was achieved within 1 month and maintained through 6 months of follow-up. However, due to significant nausea requiring antiemetics, after 5 months on DRV/c/FTC/TAF, they switched to an NNRTI (doravirine)-based regimen and stopped GAHT. In addition to more tolerable regimens, for patients such as this who desire injectable ART but have CAB resistance, novel LA-ART strategies are needed. The also merits further study. In many global settings, dolutegravir combined with TDF/3TC is first-line ART, second-line therapy may be difficult to access and less tolerable, and resistance testing is not routinely available for clinical care. Data on the impact of CAB-selected mutations on future treatment with INSTI-based ART are needed.
We assessed CAB concentrations in both plasma and hair samples to confirm ARV exposure. A random plasma CAB concentration 2 weeks after the second injection was ∼20 times above the PA-IC90 (well above the 8× PA-IC90 associated with high CAB concentrations in trials). We also analyzed CAB concentrations along the length of the hair segments, reflecting drug exposure in the weeks before and after HIV diagnosis and second CAB-LA injection, which confirmed CAB exposure. Segmental analysis is useful in cases of PrEP breakthrough in routine care when stored samples may not be available for retrospective drug level testing [12–14]. Notably, the precise time to onset of protection after first CAB-LA injection remains uncertain and could be influenced by interindividual variability in PK.
This case highlights several challenges with CAB-LA implementation in routine clinical settings that differ from trials. After 7 months of CAB-LA use, the participant lost health insurance and had a lapse in injections for 6 months. After re-establishing care, as noted above, CAB-LA had to be ordered and obtained in clinic and the patient had to return to the clinic, contributing to a gap of 20 days between initial HIV RNA testing and the first CAB-LA injection. Because this patient did not wish to take oral pills, this period was not covered by oral PrEP. HIV RNA testing was not repeated within 7 days of restart, and fidelity to HIV testing guidelines may pose challenges in terms of logistics and costs. Interruptions in insurance and health care are well-documented barriers to oral PrEP care and have been associated with seroconversion on oral PrEP [28, 29]. We now risk repeating these barriers with CAB-LA rollout. Ensuring equitable and ongoing access to CAB-LA, particularly for persons with social and structural barriers, and reducing barriers in insurance and access to care will be critical to ensuring the success of CAB-LA PrEP [30].
Strengths of this study include the comprehensive assessment with sensitive diagnostic, resistance, and PK assays and partner ARV resistance results to complement clinical and behavioral data. Additionally, this study is among the first to assess virologic outcomes on ART following HIV infection on CAB-LA and documents only the second reported HIV infection on CAB-LA in routine care. A partnership between the NCCC and the SeroPrEP study allowed for provision of clinical consultation on patient management by the NCCC, followed by referral for research testing in SeroPrEP. Limitations include the lack of stored samples from before HIV diagnosis (as is common in routine clinical care).
Long-acting cabotegravir is highly effective for HIV prevention and holds promise to reduce HIV incidence. As access expands, systems are needed to monitor and evaluate HIV acquisition in the context of CAB-LA use, including testing strategies to detect acute infections, reasons for injection delays, and sensitive testing for PK and resistance. Moreover, the impact of INSTI resistance selected by CAB on virologic outcomes in patients on first-line INSTI-based ART and optimal ART selection are pressing questions during CAB-LA rollout. Accelerated, equitable, and sustained access to CAB-LA, coupled with comprehensive HIV testing and care pathways to minimize the time between CAB-LA choice and start, is imperative to achieve the potential of CAB-LA to reduce global HIV incidence.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Acknowledgments
The SeroPrEP study is deeply grateful to the participants enrolled in the study, particularly the participant described in this manuscript. We acknowledge the late Dr. Dawn Smith of the CDC and the late Dr. Ron Goldschmidt of the NCCC for their vision with facilitating the NCCC-SeroPrEP collaboration; Dr. Mary Tanner and Dr. Jeff Johnson of the CDC; Kathleen Poortinga of the LA County Department of Public Health; James Camarillo of Monogram Biosciences for clinical genotyping; and Dr. Robert Grant, who founded the SeroPrEP study.
Author contributions. C.A.K., M.G., D.V.G., A.A.C., J.W.M., and U.M.P. contributed to study design. C.A.K., M.G., C.C., L.G.G., A.A.C., M.T., K.K., and A.M. contributed to data collection. C.A.K., M.G., E.K.H., H.O., L.G.G., A.L.H., A.A.C., C.S., D.H., K.K., A.L., H.R.G., E.W.M., K.J.P., J.W.M., and U.M.P. contributed to data analysis. C.A.K., M.G., E.K.H., H.O., C.C., D.V.G., L.G.G., A.L.H., A.A.C., C.S., D.H., K.K., A.L., H.R.G., E.W.M., K.J.P., B.H.C., J.O.A., R.P.P., J.W.M., and U.M.P. contributed to data interpretation. C.A.K., E.K.H., L.G.G., A.L.H., J.W.M., and U.M.P. contributed to preparation of figures and tables. C.A.K., M.G., E.K.H., H.O., C.C., D.V.G., L.G.G., A.L.H., A.A.C., M.T., C.S., D.H., K.K., A.L., H.R.G., E.W.M., K.J.P., B.H.C., J.O.A., A.M., R.P.P., J.W.M., and U.M.P. contributed to writing of the manuscript. C.A.K., M.G., D.V.G., A.A.C., B.H.C., J.O.A., J.W.M., and U.M.P. contributed to funding acquisition.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases at the National Institutes of Health (R01AI167753 to C.A.K. and U.M.P. and P30AI027763 to M.G.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Contributor Information
Catherine A Koss, University of California, San Francisco, San Francisco, California, USA.
Monica Gandhi, University of California, San Francisco, San Francisco, California, USA.
Elias K Halvas, University of Pittsburgh, Pittsburgh, Pennsylvania, USA.
Hideaki Okochi, University of California, San Francisco, San Francisco, California, USA.
Carolyn Chu, University of California, San Francisco, San Francisco, California, USA.
David V Glidden, University of California, San Francisco, San Francisco, California, USA.
Lisa Georgetti Gomez, University of California, San Francisco, San Francisco, California, USA.
Amy L Heaps, University of Pittsburgh, Pittsburgh, Pennsylvania, USA.
Amy A Conroy, University of California, San Francisco, San Francisco, California, USA.
Michael Tran, Men's Health Foundation, Los Angeles, California, USA.
Cory Shetler, University of Pittsburgh, Pittsburgh, Pennsylvania, USA.
Dianna Hoeth, University of Pittsburgh, Pittsburgh, Pennsylvania, USA.
Karen Kuncze, University of California, San Francisco, San Francisco, California, USA.
Alexander Louie, University of California, San Francisco, San Francisco, California, USA.
Hana Rivera Garza, University of California, San Francisco, San Francisco, California, USA.
Erick Wafula Mugoma, Global Programs for Research and Training, Nairobi, Kenya.
Kerri J Penrose, University of Pittsburgh, Pittsburgh, Pennsylvania, USA.
Bhavna H Chohan, University of Washington, Seattle, Washington, USA.
James O Ayieko, Centre for Microbiology Research, Kenya Medical Research Institute, Nairobi, Kenya.
Anthony Mills, Men's Health Foundation, Los Angeles, California, USA.
Rupa R Patel, Whitman Walker Health, Washington DC, USA; Washington University, St. Louis, Missouri, USA.
John W Mellors, University of Pittsburgh, Pittsburgh, Pennsylvania, USA.
Urvi M Parikh, University of Pittsburgh, Pittsburgh, Pennsylvania, USA.
References
- 1. Landovitz RJ, Hanscom BS, Clement ME, et al. Efficacy and safety of long-acting cabotegravir compared with daily oral tenofovir disoproxil fumarate plus emtricitabine to prevent HIV infection in cisgender men and transgender women who have sex with men 1 year after study unblinding: a secondary analysis of the phase 2b and 3 HPTN 083 randomised controlled trial. Lancet HIV 2023; 10:e767–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Landovitz RJ, Donnell D, Clement ME, et al. Cabotegravir for HIV prevention in cisgender men and transgender women. N Engl J Med 2021; 385:595–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Delany-Moretlwe S, Hughes JP, Bock P, et al. Cabotegravir for the prevention of HIV-1 in women: results from HPTN 084, a phase 3, randomised clinical trial. Lancet 2022; 399:1779–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. World Health Organization . Guidelines on Long-Acting Injectable Cabotegravir for HIV Prevention. World Health Organization; 2022. [PubMed] [Google Scholar]
- 5. Eshleman SH, Fogel JM, Piwowar-Manning E, et al. Characterization of human immunodeficiency virus (HIV) infections in women who received injectable cabotegravir or tenofovir disoproxil fumarate/emtricitabine for HIV prevention: HPTN 084. J Infect Dis 2022; 225:1741–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Marzinke MA, Fogel JM, Wang Z, et al. Extended analysis of HIV infection in cisgender men and transgender women who have sex with men receiving injectable cabotegravir for HIV prevention: HPTN 083. Antimicrob Agents Chemother 2023; 67:e0005323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Parikh UM, Koss CA, Mellors JW. Long-acting injectable cabotegravir for HIV prevention: what do we know and need to know about the risks and consequences of cabotegravir resistance? Curr HIV/AIDS Rep 2022; 19:384–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hazra A, Landovitz RJ, Marzinke MA, Quinby C, Creticos C. Breakthrough HIV-1 infection in setting of cabotegravir for HIV pre-exposure prophylaxis. AIDS 2023; 37:1711–4. [DOI] [PubMed] [Google Scholar]
- 9. Eshleman SH, Fogel JM, Halvas EK, et al. HIV RNA screening reduces integrase strand transfer inhibitor resistance risk in persons receiving long-acting cabotegravir for HIV prevention. J Infect Dis 2022; 226:2170–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med 1998; 158:1789–95. [DOI] [PubMed] [Google Scholar]
- 11. Thaden JT, Gandhi M, Okochi H, Hurt CB, McKellar MS. Seroconversion on preexposure prophylaxis: a case report with segmental hair analysis for timed adherence determination. AIDS 2018; 32:F1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cohen SE, Sachdev D, Lee SA, et al. Acquisition of tenofovir-susceptible, emtricitabine-resistant HIV despite high adherence to daily pre-exposure prophylaxis: a case report. Lancet HIV 2018; S2352-3018(18)30288-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Colby DJ, Kroon E, Sacdalan C, et al. Acquisition of multidrug-resistant human immunodeficiency virus type 1 infection in a patient taking preexposure prophylaxis. Clin Infect Dis 2018; 67:962–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Spinelli MA, Lowery B, Shuford JA, et al. Use of drug-level testing and single-genome sequencing to unravel a case of human immunodeficiency virus seroconversion on pre-exposure prophylaxis. Clin Infect Dis 2021; 72:2025–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Saberi P, Mehtani NJ, Sayegh A, Camp CE, Chu C. Understanding HIV pre-exposure prophylaxis questions of U.S. health care providers: unique perspectives from the PrEPline clinical teleconsultation service. Telemed J E Health 2023; 29:376–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. US Public Health Service . Preexposure Prophylaxis for the Prevention of HIV Infection in the United States—2021 Update: A Clinical Practice Guideline. US Public Health Service; 2021. [Google Scholar]
- 17. Jacobs JL, Tosiano MA, Koontz DL, et al. Automated multireplicate quantification of persistent HIV-1 viremia in individuals on antiretroviral therapy. J Clin Microbiol 2020; 58:e01442–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gandhi M, Louie A, Spinelli M, et al. Paper presented at: Conference on Retroviruses and Opportunistic Infections; 2024; Denver, Colorado, USA, March 3-6, 2024.
- 19. World Health Organization . Consolidated Guidelines on HIV, Viral Hepatitis and STI Prevention, Diagnosis, Treatment and Care for Key Populations. World Health Organization; 2022. [PubMed] [Google Scholar]
- 20. Landovitz R, Voldal E, Hanscom B, et al. Paper presented at: Conference on Retroviruses and Opportunistic Infections; 2024; Denver, Colorado, USA, March 3-6, 2024.
- 21. Zhu W, Huang YA, Delaney KP, Patel R, Kourtis A, Hoover KW. Paper presented at: Conference on Retroviruses and Opportunistic Infections; 2024; Denver, Colorado, USA, March 3-6, 2024.
- 22. California Prevention Training Center . Injectable PrEP frequently asked questions—clinical. 2024. Available at: https://californiaptc.com/prep-learning-collaboratives/long-acting-injectable-prep/injectable-prep-frequently-asked-questions/. Accessed February 24, 2024.
- 23. Rhee SY, Parkin N, Harrigan PR, Holmes S, Shafer RW. Genotypic correlates of resistance to the HIV-1 strand transfer integrase inhibitor cabotegravir. Antiviral Res 2022; 208:105427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Monogram Biosciences . GenoSure PRIme. Available at: https://monogrambio.labcorp.com/resources/genotyping/genosure-prime. Accessed March 12, 2024.
- 25. McClung RP, Oster AM, Ocfemia MCB, et al. Transmitted drug resistance among human immunodeficiency virus (HIV)-1 diagnoses in the United States, 2014–2018. Clin Infect Dis 2022; 74:1055–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Loosli T, Hossmann S, Ingle SM, et al. HIV-1 drug resistance in people on dolutegravir-based antiretroviral therapy: a collaborative cohort analysis. Lancet HIV 2023; 10:e733–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Panel on Antiretroviral Guidelines for Adults and Adolescent . Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents With HIV. Department of Health and Human Services; 2023. [Google Scholar]
- 28. Patel RR, Mena L, Nunn A, et al. Impact of insurance coverage on utilization of pre-exposure prophylaxis for HIV prevention. PLoS One 2017; 12:e0178737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Marcus JL, Hurley LB, Dentoni-Lasofsky D, et al. Barriers to preexposure prophylaxis use among individuals with recently acquired HIV infection in Northern California. AIDS Care 2019; 31:536–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Patel RR. Paper presented at: Conference on Retroviruses and Opportunistic Infections; 2024; Denver, Colorado, USA, March 3-6, 2024.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.